
Draw the resonating structure of ${{O}_{3}}$ and $C{{O}_{3}}$.
Answer
588k+ views
Hint: Ozone (${{O}_{3}}$ ) do not have any overall charge but carbonate has an overall charge of -2. One of the oxygen atoms is negatively charged and one is positively charged in one of the resonating structures of ozone.
Complete step by step solution:
Resonating structures are the structures that some compounds may show by change in the positions of double bonds. These resonating structures give the compounds more stability.
- The charge on the atoms may change in the resonating structures. As example an atom may bear the charge in one of the resonating structures but it is not necessary that it will bear the charge on other structures as well.
Let’s see the resonating structures of Ozone first.
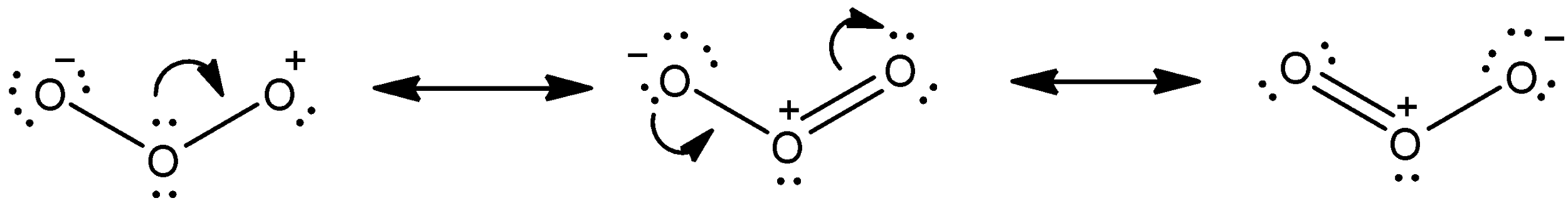
We know that ozone consists of three oxygen atoms and oxygen atoms can make bonds with two other atoms as it is bivalent. Now, in one structure (first one), we can see that one of the oxygen atoms is negatively charged and one has positive charge. Now, the central atom can donate its electron pair and make a double bond with the oxygen that has positive charge. So, the central atom will have positive charge. Then, the negatively charged atom can form double bond with the central atom which is positively charged (we can see that in the second structure) and as a result the neutral oxygen atom will become negatively charged.
- Thus, these are the three resonating structures of Ozone.
Resonating structures of Carbonate:
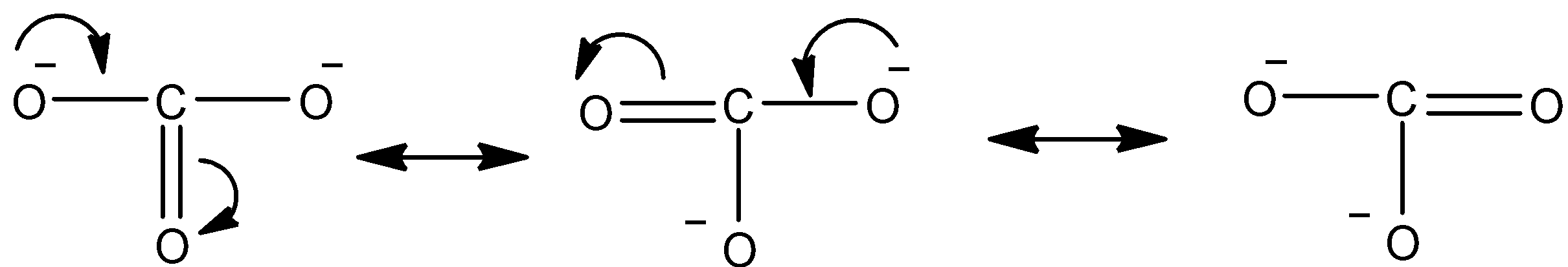
In carbonate, two oxygen atoms have negative charge and one oxygen atom is doubly bonded to the carbon atom. Thus, carbonate has an overall charge of -2. The negatively charged oxygen atom will donate its extra one pair and make a double bond with the carbon and simultaneously the existing double bond will be broken. Same way both negatively charged oxygen atoms can form double bonds and give the resonating structures.
Note: Remember while forming and breaking the double bonds that oxygen atoms cannot have negative charge while it is bonded with three atoms or has three bonds. It will always have positive charge when it is bonded with three other atoms or makes three bonds.
Complete step by step solution:
Resonating structures are the structures that some compounds may show by change in the positions of double bonds. These resonating structures give the compounds more stability.
- The charge on the atoms may change in the resonating structures. As example an atom may bear the charge in one of the resonating structures but it is not necessary that it will bear the charge on other structures as well.
Let’s see the resonating structures of Ozone first.

We know that ozone consists of three oxygen atoms and oxygen atoms can make bonds with two other atoms as it is bivalent. Now, in one structure (first one), we can see that one of the oxygen atoms is negatively charged and one has positive charge. Now, the central atom can donate its electron pair and make a double bond with the oxygen that has positive charge. So, the central atom will have positive charge. Then, the negatively charged atom can form double bond with the central atom which is positively charged (we can see that in the second structure) and as a result the neutral oxygen atom will become negatively charged.
- Thus, these are the three resonating structures of Ozone.
Resonating structures of Carbonate:

In carbonate, two oxygen atoms have negative charge and one oxygen atom is doubly bonded to the carbon atom. Thus, carbonate has an overall charge of -2. The negatively charged oxygen atom will donate its extra one pair and make a double bond with the carbon and simultaneously the existing double bond will be broken. Same way both negatively charged oxygen atoms can form double bonds and give the resonating structures.
Note: Remember while forming and breaking the double bonds that oxygen atoms cannot have negative charge while it is bonded with three atoms or has three bonds. It will always have positive charge when it is bonded with three other atoms or makes three bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




