
Draw the resonating structure of:
A. Ozone molecule
B. Nitrate ion
Answer
541.8k+ views
Hint: Resonance is a way to explain delocalized electrons inside several molecules or polyatomic ions where a single Lewis formula does not convey the bonding. Several resonance structures are represented by a molecule or ion with such delocalized electrons.
Complete answer:
Resonance structures are two examples of a molecule in which the chemical interaction is the same, but the electrons are distributed around the differential structure.
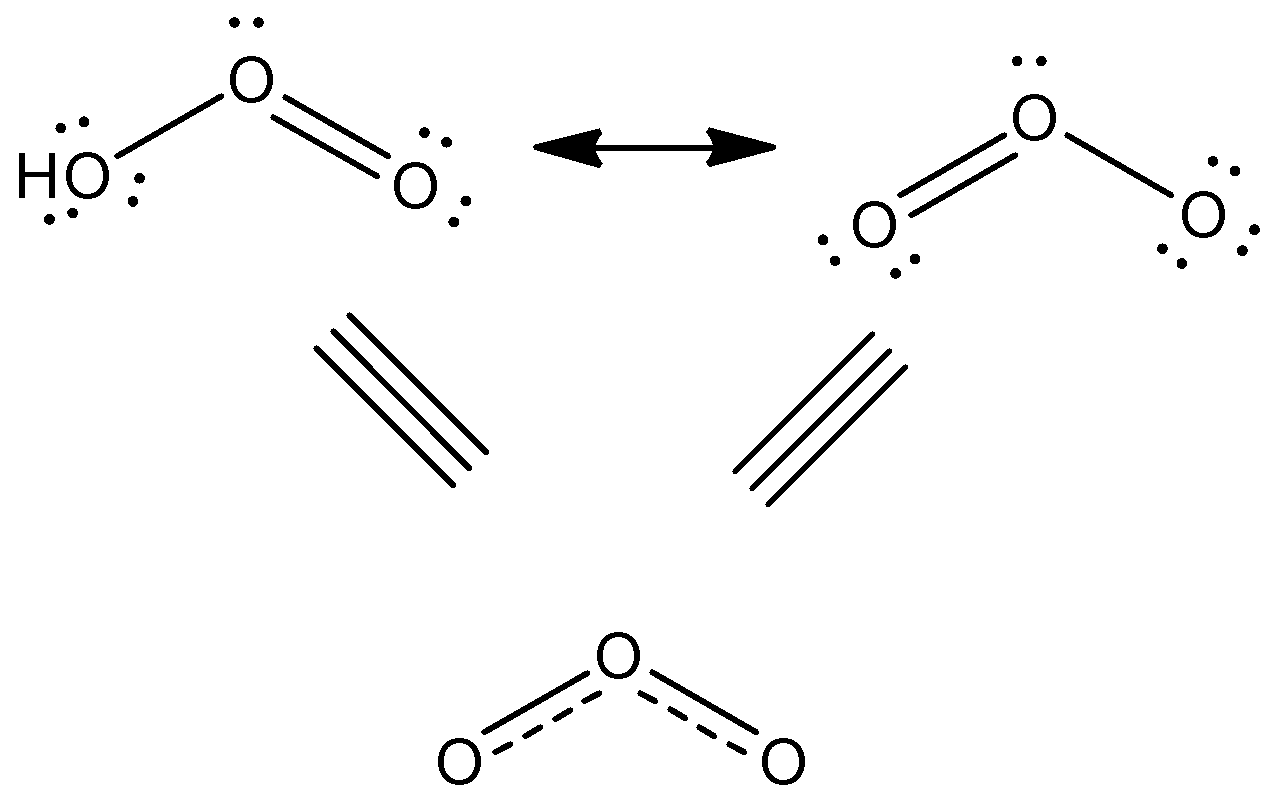
There are two main resonance structures in ozone or ${{O}_{3}}$ that contribute similarly to the overall hybrid structure of the molecule. All structures represent the 18 required valence electrons-6 out of 3 bonds and 12 as lone pairs placed on the oxygen atoms.
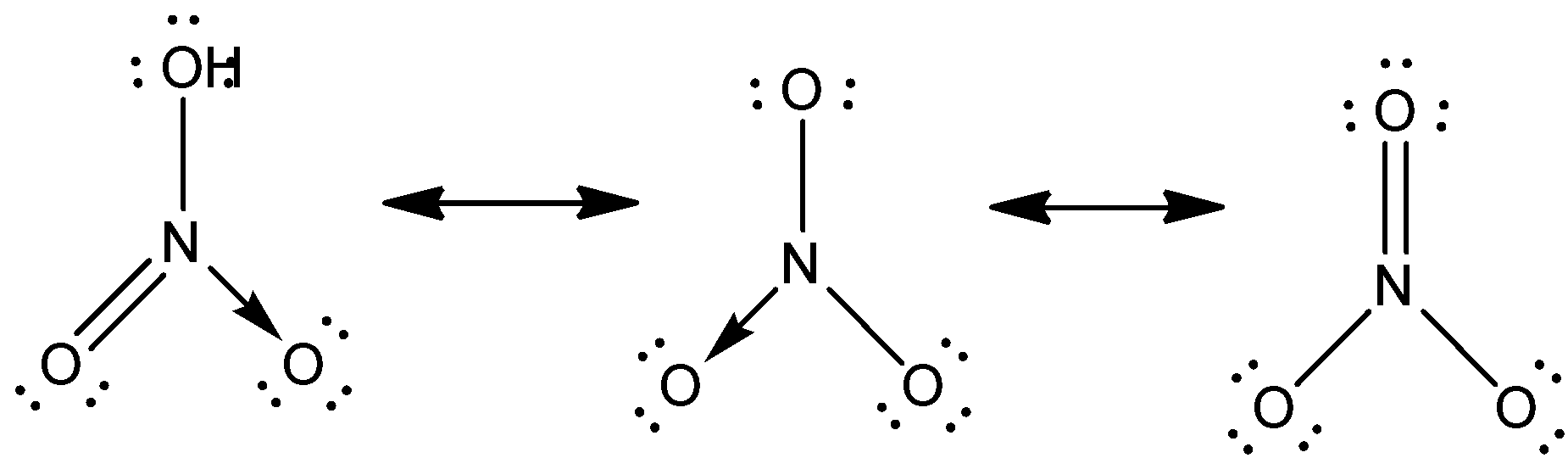
According to the principle of resonance, the nitrate ion structure is not 1 or 2 or 3, but the stability-weighted average of all three of them. There are two $\pi $ electrons in each resonance form of the nitrate ion, and they are shared only by two atoms. It is said that an electron shared only by two atoms is localised.
Note:
Resonance is a way to describe in some molecules or ions the combination of several contributing structures (or forms, also known as resonance structures or canonical structures) into a hybrid resonance (or hybrid structure) in valence bond theory. If the frequency matches the resonant frequency of the object it reaches, you will get what is known as resonance.
Complete answer:
Resonance structures are two examples of a molecule in which the chemical interaction is the same, but the electrons are distributed around the differential structure.

There are two main resonance structures in ozone or ${{O}_{3}}$ that contribute similarly to the overall hybrid structure of the molecule. All structures represent the 18 required valence electrons-6 out of 3 bonds and 12 as lone pairs placed on the oxygen atoms.

According to the principle of resonance, the nitrate ion structure is not 1 or 2 or 3, but the stability-weighted average of all three of them. There are two $\pi $ electrons in each resonance form of the nitrate ion, and they are shared only by two atoms. It is said that an electron shared only by two atoms is localised.
Note:
Resonance is a way to describe in some molecules or ions the combination of several contributing structures (or forms, also known as resonance structures or canonical structures) into a hybrid resonance (or hybrid structure) in valence bond theory. If the frequency matches the resonant frequency of the object it reaches, you will get what is known as resonance.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




