
Draw the more stable chair conformer of cis-1-ethyl-2-methylcyclohexane
Answer
557.7k+ views
Hint: We could say that conformations of cyclohexane are any of a few three-dimensional shapes adopted by cyclohexane molecules. Since numerous mixes highlight basically comparable six-membered rings, the structure and elements of cyclohexane are significant models of a wide scope of compounds.
Complete step by step answer:
We have to draw the wedge-dash notation for cis-1-ethyl-2-methylcyclohexane. The structure could be given as,
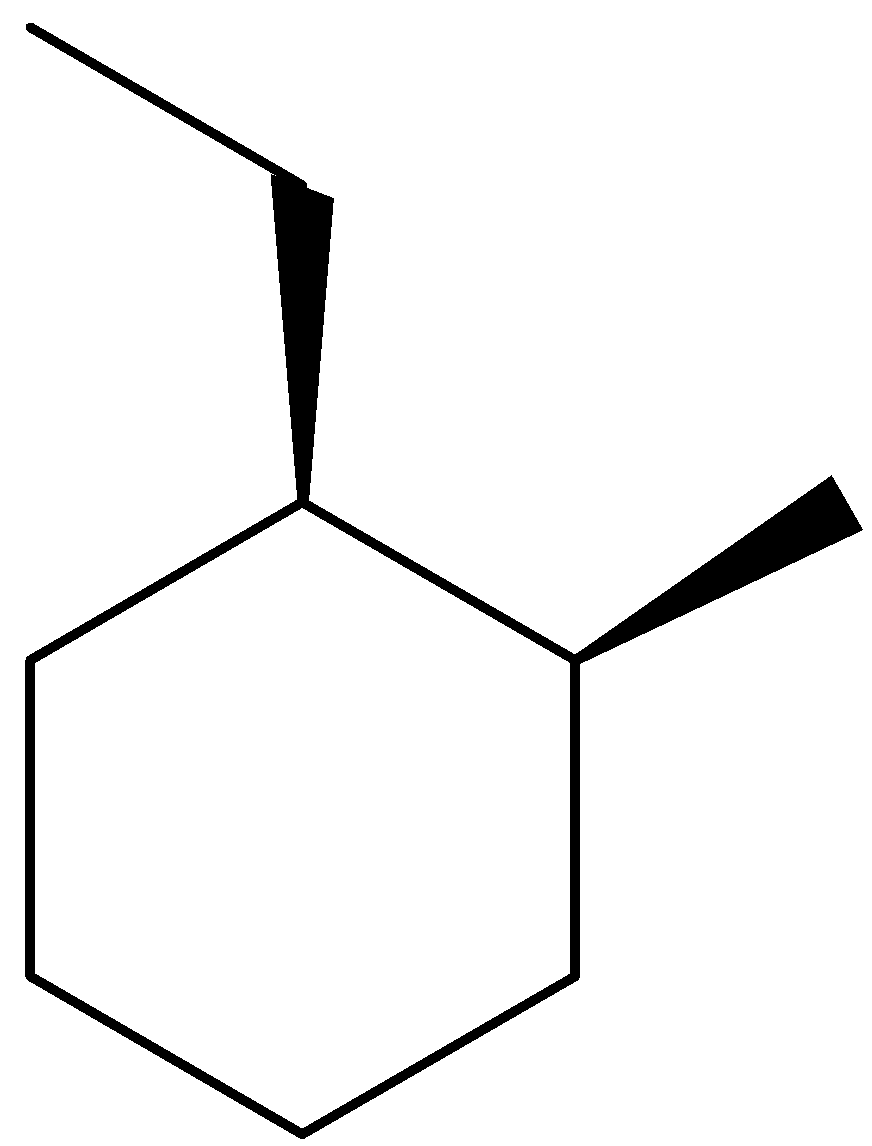
Because the methyl group and ethyl group are in cis relationship, both must be keep either on dashes (or) on wedges.
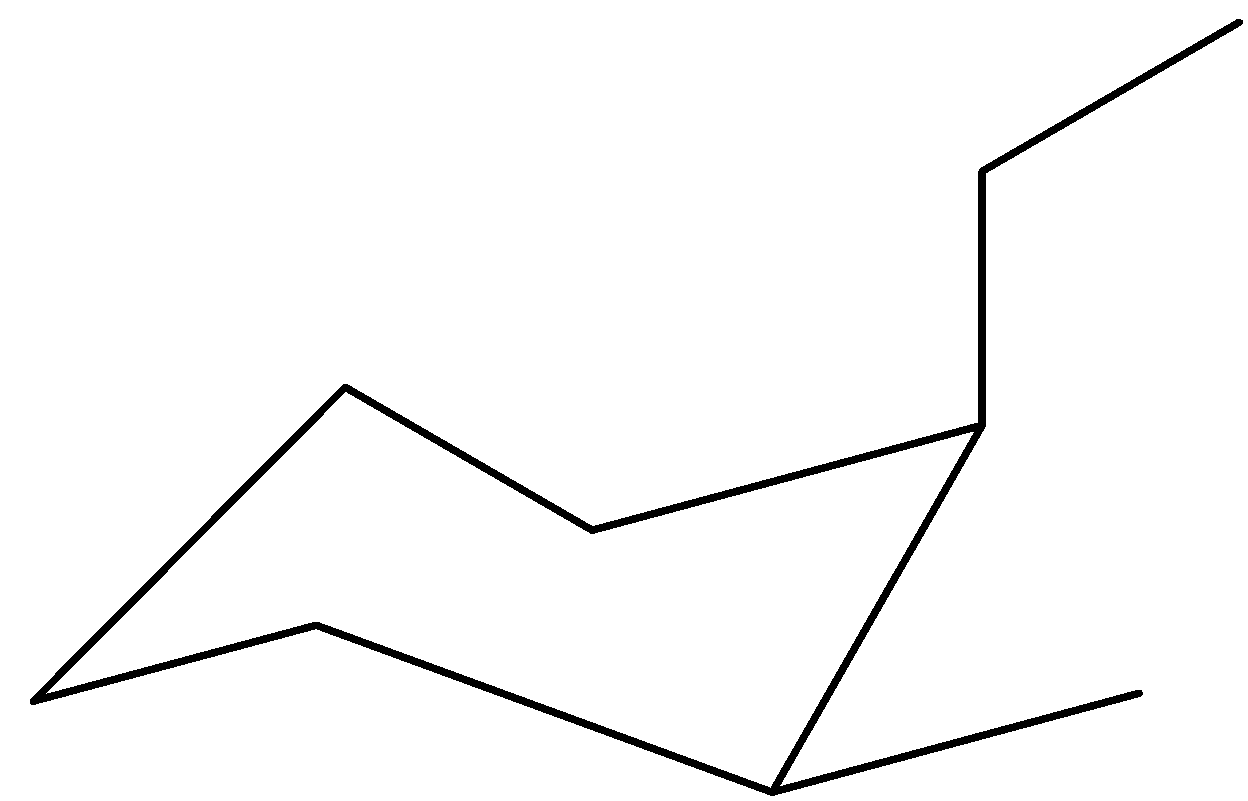
We can draw the first chair confirmation by placing the ethyl group upwards on the axial bond and the methyl group upwards on equatorial bonds. This would be the placement of the groups as if we go around the ring, the same orientation groups would fall on alternating axial and equatorial bonds.
To determine the most stable chair of this molecule, we have to draw the chair flip conformer also. First draw the empty chain flip.

Now, when chair flip is flipped, we have to remember that orientation does not change. Upwards would still be UP and down would still be down; but axial bonds would turn equatorial and vice versa.
This says that the ethyl group would go from being upwards on an axial bond to turning up on an equatorial bond. The same thing would take place with the methyl group, only this time up equatorial would become up axial.
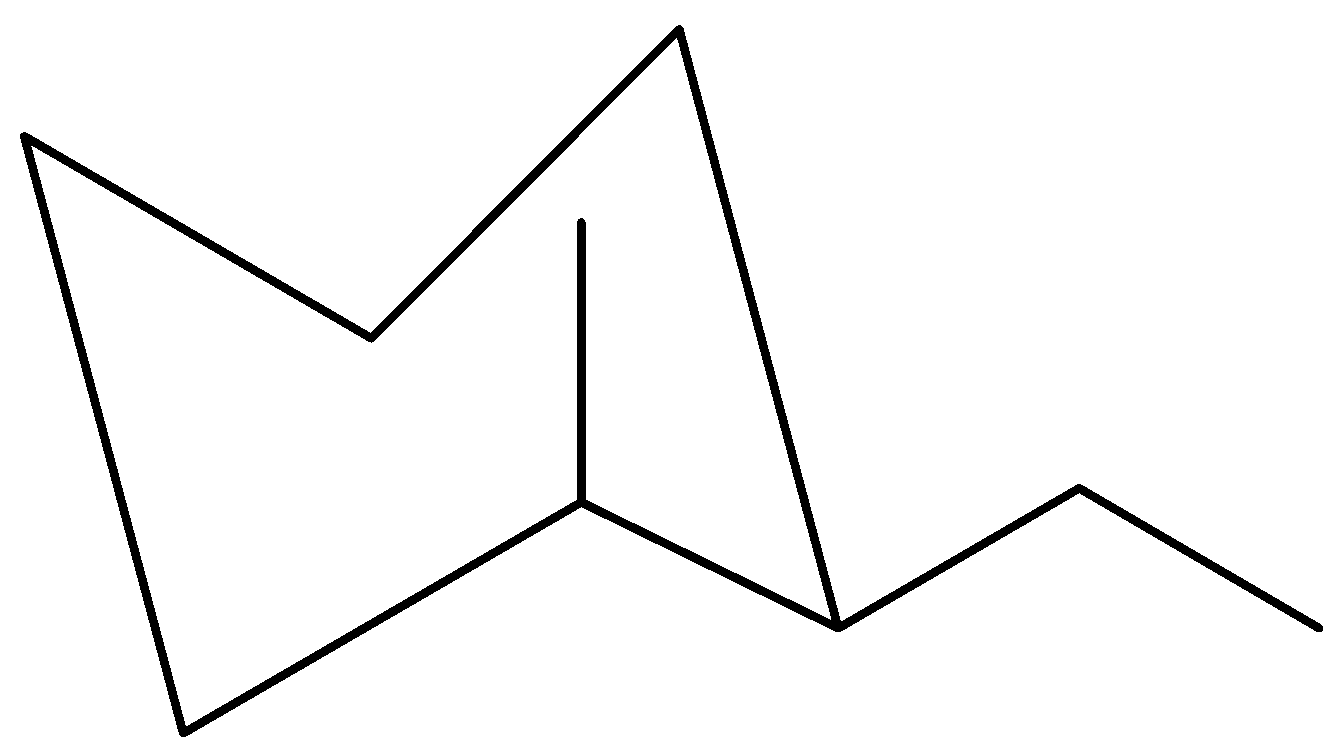
Let us now find out the most stable conformer. For cyclohexane chair conformations, stability would be based on what kinds of bonds (axial or equatorial) the larger groups are positioned. Ideally, methyl as well as ethyl groups must be on equatorial bonds for the chair conformation to be highly stable.
In this case, they are alternating among being on axial and on equatorial bonds. As a result, the most stable chair conformer would contain the largest group on an equatorial bond that means the second chair (the flipped one) is the more stable of the two.
Note: We need to remember that ${D_{3d}}$ is the symmetry of chair conformation of cyclohexane. The interconversion of chain conformers is known as ring flipping or seat flipping. Bonds of carbon-hydrogen that are axial in one configuration become equatorial in the other, and the other way around. At room temperature, the proton NMR range of cyclohexane is a singlet.
Complete step by step answer:
We have to draw the wedge-dash notation for cis-1-ethyl-2-methylcyclohexane. The structure could be given as,

Because the methyl group and ethyl group are in cis relationship, both must be keep either on dashes (or) on wedges.
We can draw the first chair confirmation by placing the ethyl group upwards on the axial bond and the methyl group upwards on equatorial bonds. This would be the placement of the groups as if we go around the ring, the same orientation groups would fall on alternating axial and equatorial bonds.

To determine the most stable chair of this molecule, we have to draw the chair flip conformer also. First draw the empty chain flip.

Now, when chair flip is flipped, we have to remember that orientation does not change. Upwards would still be UP and down would still be down; but axial bonds would turn equatorial and vice versa.
This says that the ethyl group would go from being upwards on an axial bond to turning up on an equatorial bond. The same thing would take place with the methyl group, only this time up equatorial would become up axial.

Let us now find out the most stable conformer. For cyclohexane chair conformations, stability would be based on what kinds of bonds (axial or equatorial) the larger groups are positioned. Ideally, methyl as well as ethyl groups must be on equatorial bonds for the chair conformation to be highly stable.
In this case, they are alternating among being on axial and on equatorial bonds. As a result, the most stable chair conformer would contain the largest group on an equatorial bond that means the second chair (the flipped one) is the more stable of the two.
Note: We need to remember that ${D_{3d}}$ is the symmetry of chair conformation of cyclohexane. The interconversion of chain conformers is known as ring flipping or seat flipping. Bonds of carbon-hydrogen that are axial in one configuration become equatorial in the other, and the other way around. At room temperature, the proton NMR range of cyclohexane is a singlet.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




