
Draw the Lewis structure for the molecule \[C{H_2}CHC{H_3}\] . How many times sigma and pi bonds does it contain?
Answer
557.7k+ views
Hint: A Lewis structure is represented by the valence shell electrons in a molecule. The Lewis structure is used to show how the electrons are arranged around an individual atom in the molecule. Electrons are shown in the form of "dots" or for bonding electrons as a line between the two atoms. The aim is to obtain the "best" electron configuration, i.e., the octet rule and formal charges need to be fulfilled.
Complete step-by-step answer:Propylene is also known as propene or methyl ethylene. It is an unsaturated organic compound which has one double bond and remaining bonds are single-bond. This is the second simplest member of the alkene class of hydrocarbons. It is a colourless gas with a faint petroleum-like smell. Bio-propylene is the bio-based counterpart of propene.
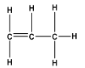
The number of sigma and pi-bonds are eight and one respectively.
Explanation:
Lewis-dot structure: It shows the bonding between the atoms of a molecule as shown in the structure and it also shows the unpaired electrons present in the molecule.
In the Lewis-dot structure the valence electrons are represented by 'dot'.
The given molecule is propylene, \[C{H_2}CHC{H_3}\] .
As we know that carbon has four valence electrons and hydrogen has one valence electron.
Therefore, the total number of valence electrons in propene will be;
\[C{H_2}CHC{H_3}\, = \,3(4)\, + \,6(1)\, = 18\]
According to the Lewis-dot structure, there are \[18\] number of bonding electrons and \[0\] number of nonbonding electrons.
The number of \[8\] \[\sigma \] -bonds and \[1\] \[\pi \] -bond and respectively.

Note:In the propene molecule, \[{H_2}C = CH - C{H_3}\] , there is one \[\pi \] -bond (i.e., \[C = C\] bond), eight \[\sigma \] -bonds (i.e., \[7\;C - H\] and \[C - C\;\sigma \;\] -bonds). There are \[1 \times C - C\] bonds, \[1 \times C = C\;\] bonds, and \[\;6 \times C - H\;\] bonds. The Lewis structure makes it simpler to represent the structure and identify the sigma and pi- bonds.
Complete step-by-step answer:Propylene is also known as propene or methyl ethylene. It is an unsaturated organic compound which has one double bond and remaining bonds are single-bond. This is the second simplest member of the alkene class of hydrocarbons. It is a colourless gas with a faint petroleum-like smell. Bio-propylene is the bio-based counterpart of propene.

The number of sigma and pi-bonds are eight and one respectively.
Explanation:
Lewis-dot structure: It shows the bonding between the atoms of a molecule as shown in the structure and it also shows the unpaired electrons present in the molecule.
In the Lewis-dot structure the valence electrons are represented by 'dot'.
The given molecule is propylene, \[C{H_2}CHC{H_3}\] .
As we know that carbon has four valence electrons and hydrogen has one valence electron.
Therefore, the total number of valence electrons in propene will be;
\[C{H_2}CHC{H_3}\, = \,3(4)\, + \,6(1)\, = 18\]
According to the Lewis-dot structure, there are \[18\] number of bonding electrons and \[0\] number of nonbonding electrons.
The number of \[8\] \[\sigma \] -bonds and \[1\] \[\pi \] -bond and respectively.

Note:In the propene molecule, \[{H_2}C = CH - C{H_3}\] , there is one \[\pi \] -bond (i.e., \[C = C\] bond), eight \[\sigma \] -bonds (i.e., \[7\;C - H\] and \[C - C\;\sigma \;\] -bonds). There are \[1 \times C - C\] bonds, \[1 \times C = C\;\] bonds, and \[\;6 \times C - H\;\] bonds. The Lewis structure makes it simpler to represent the structure and identify the sigma and pi- bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




