
Draw the Lewis dot structure for $N{{O}_{2}}^{-}$.
Answer
566.1k+ views
Hint: Lewis dot structure, also known as electron dot structure, is the representation of the valence electrons of atoms within a molecule. It helps us to visualize the valence electrons of atoms and molecules, the formal charge of individual elements and if they exist as lone pairs or within bonds.
Complete step by step answer:
The compound is called Nitrite ion.
While drawing a Lewis dot structure, we need to follow certain steps –
Select the central atom.
- In nitrite ion, only one nitrogen is present. It therefore, is the central atom.
- Complete the octet of the corner atoms first. (in this case, oxygen)
- Oxygen has 6 electrons in its valence shell, we can represent non-bonding electrons in the form of lone pairs.
Complete the octet of central atom (in this case, nitrogen)
- Nitrogen has 5 electrons in its valence, in order to complete its octet, it forms 3 bonds and has 1 lone pair of electrons.
The structure now becomes –
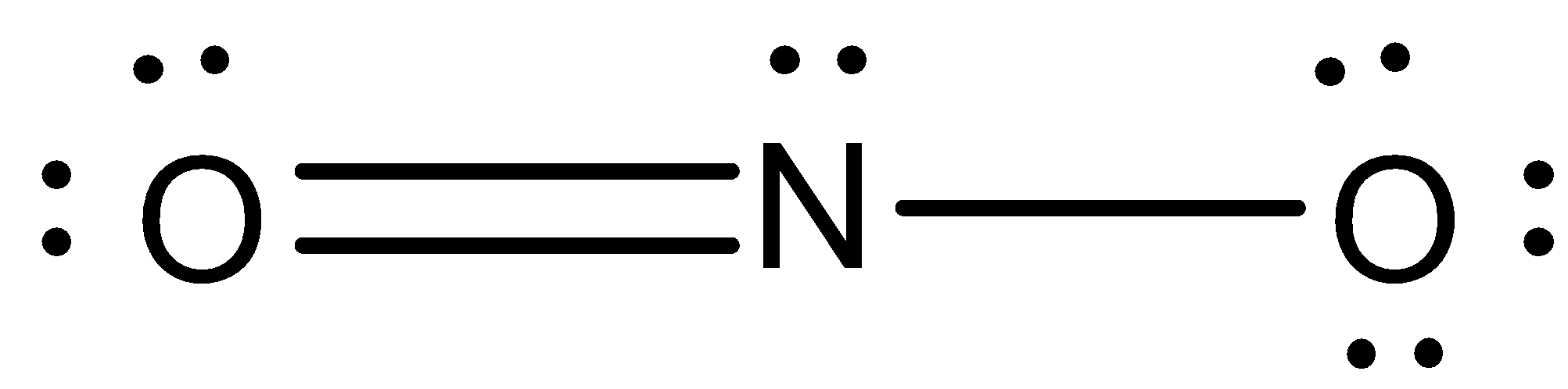
As we can see, the elements are not balanced. Therefore, now we will calculate the formal charge on each compound. Formal charge is the charge on an individual atom.
Formal charge = Valence electron – Lone pair electron – (Bond pair electron/2).
Therefore,
Formal charge on Nitrogen = $5 - 2 - \dfrac{6}{2} = 0$
Formal charge on Oxygen 1 = $6 - 4 - \dfrac{4}{2} = 0$
Formal charge on Oxygen 2 = $6 - 6 - \dfrac{2}{2} = -1$
Therefore, the Lewis structure of Nitrile ion is represented as –
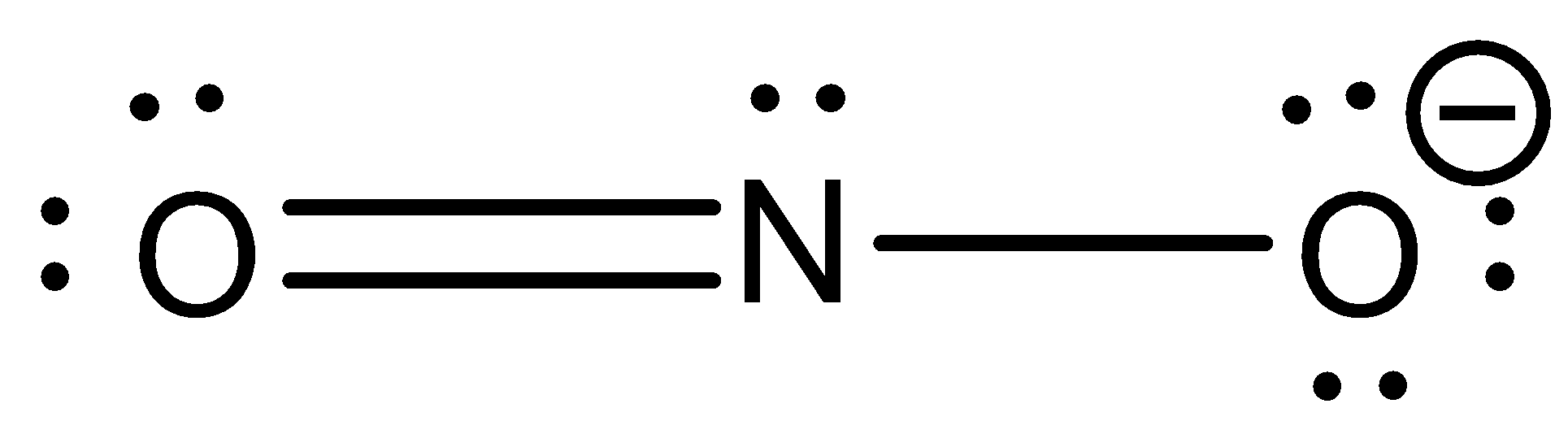
Additional Information: A negatively charged compound is an anion, whereas, a positively charged compound is a cation. The compound given in this question is an anion.
Note: There are certain points which need to be kept in mind while drawing a Lewis dot structure. The central atom must follow one or more of the given criterions -
- either has minimum number of atoms
- has the least electron affinity
- has the largest size
- highest atomic number
- Hydrogen and Fluorine can never be the central atom.
Complete step by step answer:
The compound is called Nitrite ion.
While drawing a Lewis dot structure, we need to follow certain steps –
Select the central atom.
- In nitrite ion, only one nitrogen is present. It therefore, is the central atom.
- Complete the octet of the corner atoms first. (in this case, oxygen)
- Oxygen has 6 electrons in its valence shell, we can represent non-bonding electrons in the form of lone pairs.
Complete the octet of central atom (in this case, nitrogen)
- Nitrogen has 5 electrons in its valence, in order to complete its octet, it forms 3 bonds and has 1 lone pair of electrons.
The structure now becomes –
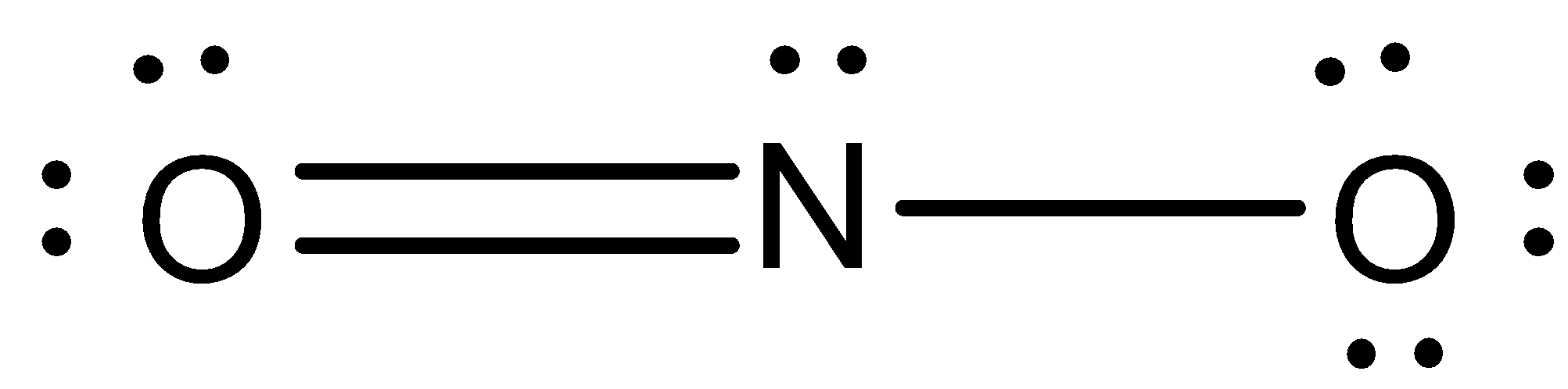
As we can see, the elements are not balanced. Therefore, now we will calculate the formal charge on each compound. Formal charge is the charge on an individual atom.
Formal charge = Valence electron – Lone pair electron – (Bond pair electron/2).
Therefore,
Formal charge on Nitrogen = $5 - 2 - \dfrac{6}{2} = 0$
Formal charge on Oxygen 1 = $6 - 4 - \dfrac{4}{2} = 0$
Formal charge on Oxygen 2 = $6 - 6 - \dfrac{2}{2} = -1$
Therefore, the Lewis structure of Nitrile ion is represented as –
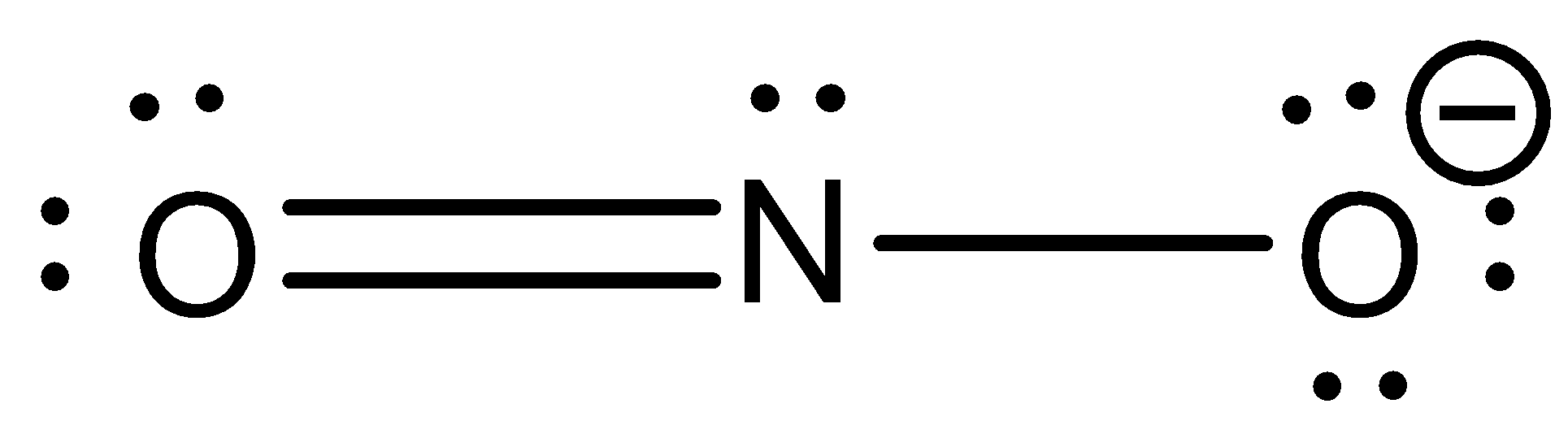
Additional Information: A negatively charged compound is an anion, whereas, a positively charged compound is a cation. The compound given in this question is an anion.
Note: There are certain points which need to be kept in mind while drawing a Lewis dot structure. The central atom must follow one or more of the given criterions -
- either has minimum number of atoms
- has the least electron affinity
- has the largest size
- highest atomic number
- Hydrogen and Fluorine can never be the central atom.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




