
How do you draw the Lewis dot structure for $KN{{O}_{3}}$ ?
Answer
565.2k+ views
Hint: The Lewis structures are the diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that can exist in the molecule. They are also known as electron dot structures. It adds up two lines between the atoms that show shared pairs.
Complete answer:
- In order to draw the Lewis structure for $KN{{O}_{3}}$, these steps are to be followed,
- First of all for nitrate ion, we will add up the valence electrons as:
5 (nitrogen electrons) + 3 X 6(oxygen electrons) + 1 negative charge = 24 electrons.
- Hence, we get 12 electron pairs that distribute to the four centres, and as nitrogen is the least electronegative atom and hence is the central atom.
- We can draw the Lewis structure for $KN{{O}_{3}}$ as:
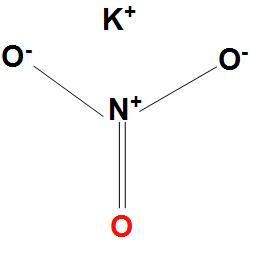
- We can see here that the nitrogen atom in nitrate has a formal positive charge. And all the oxygen atoms are equivalent.
- As nitrate has a formal negative charge according to the Lewis structure, and the neutrality for the salt is obtained with the potassium cation.
Note:
- The octet rule exists because the atoms of most of the elements become more stable by attaining the electronic configuration of an element. As we know that in octet rule atoms prefer to have eight electrons in the valence shell.
- Lewis structure mainly describes the structure of molecules with the help of symbols for the chemical species and dot symbols.
Complete answer:
- In order to draw the Lewis structure for $KN{{O}_{3}}$, these steps are to be followed,
- First of all for nitrate ion, we will add up the valence electrons as:
5 (nitrogen electrons) + 3 X 6(oxygen electrons) + 1 negative charge = 24 electrons.
- Hence, we get 12 electron pairs that distribute to the four centres, and as nitrogen is the least electronegative atom and hence is the central atom.
- We can draw the Lewis structure for $KN{{O}_{3}}$ as:

- We can see here that the nitrogen atom in nitrate has a formal positive charge. And all the oxygen atoms are equivalent.
- As nitrate has a formal negative charge according to the Lewis structure, and the neutrality for the salt is obtained with the potassium cation.
Note:
- The octet rule exists because the atoms of most of the elements become more stable by attaining the electronic configuration of an element. As we know that in octet rule atoms prefer to have eight electrons in the valence shell.
- Lewis structure mainly describes the structure of molecules with the help of symbols for the chemical species and dot symbols.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




