
Draw the electron dot structure of propanone.
Answer
503.1k+ views
Hint: The Lewis electron dot diagram is a method to represent the valence electrons of an atom with the help of dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. In a molecule, the electron dot diagram also shows the number of electrons shared while forming a chemical bond along with the valence electrons.
Complete answer:
Propanone which is commonly known as acetone is an organic compound with chemical formula $ {(C{H_3})_2}CO $ and it is the smallest and simplest ketone. Structurally it is represented as follows:
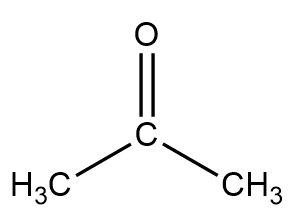
Now, to find the Lewis electron dot structure of the given molecule, we must first find out the number of valence electrons in each element in the molecule.
Valence electrons: The number of electrons present in the outermost shell of an element is referred to as valence electrons. Number of valence electrons in carbon, oxygen and hydrogen are 4, 6 and 1 respectively. Therefore, the Lewis electron dot diagram of propanone can be represented as follows:
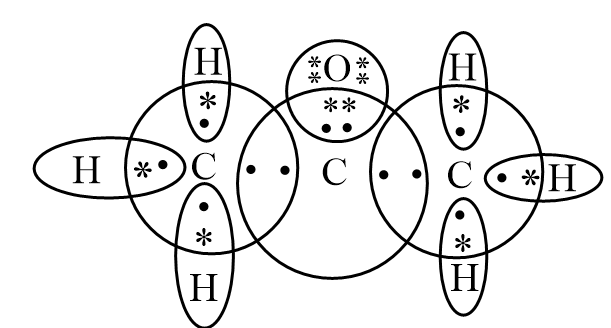
In the diagram, the valence electrons of carbon are represented by dots while the valence electrons of hydrogen and oxygen are represented by an asterisk to avoid confusion.
Note:
It is important to note that the structures drawn on the basis of the Lewis electron dot diagram reflect the fact that the elements in period 2 and beyond tend to lose, gain or share electron pairs in order to reach a total of eight valence electrons in their compound except hydrogen as it consists of only two valence electrons and does not obey octet rule.
Complete answer:
Propanone which is commonly known as acetone is an organic compound with chemical formula $ {(C{H_3})_2}CO $ and it is the smallest and simplest ketone. Structurally it is represented as follows:

Now, to find the Lewis electron dot structure of the given molecule, we must first find out the number of valence electrons in each element in the molecule.
Valence electrons: The number of electrons present in the outermost shell of an element is referred to as valence electrons. Number of valence electrons in carbon, oxygen and hydrogen are 4, 6 and 1 respectively. Therefore, the Lewis electron dot diagram of propanone can be represented as follows:

In the diagram, the valence electrons of carbon are represented by dots while the valence electrons of hydrogen and oxygen are represented by an asterisk to avoid confusion.
Note:
It is important to note that the structures drawn on the basis of the Lewis electron dot diagram reflect the fact that the elements in period 2 and beyond tend to lose, gain or share electron pairs in order to reach a total of eight valence electrons in their compound except hydrogen as it consists of only two valence electrons and does not obey octet rule.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE

Differentiate between calcination and roasting class 11 chemistry CBSE




