
Draw the electron dot structure of an ethane molecule(\[{C_2}{H_6}\] ).
Answer
575.7k+ views
Hint: Electronic dot structure is also known as Lewis dot structures and they represent the bond between the various atoms and valence electrons of atoms, within a molecule.
Complete step by step answer:
We can draw the electron dot structure if we actually know the molecule formula of the compound.
Importance of Lewis dot structures:
(a) Describe the chemical bonding between atoms in a molecule.
(b) Displace the total number of lone pairs representing each of the Atoms of molecules.
(c) Reflect the electronic structure of elements.
In Lewis dot structure, each dot represents an electron and a pair of dots between chemical symbols for atoms represents a bond.
The molecule formula of ethane is \[{C_2}{H_6}\] and the structure formula of ethane is \[C{H_3} - C{H_3}\] .
There are two carbons present in ethane molecules that are single bonded with each other and three hydrogen atoms are present with each carbon.
The electron dot structure of ethane is
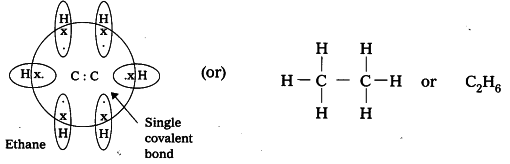
Note: Electron-dot structure also known as Lewis start structure actually shows the nature of bond and the position of atoms of the molecule that are connected in the molecule.
It is important and to be noted that only the valence electrons are considered while drawing the Lewis dot structures and the other electrons that do not belong to the outermost shell are ignored.
Complete step by step answer:
We can draw the electron dot structure if we actually know the molecule formula of the compound.
Importance of Lewis dot structures:
(a) Describe the chemical bonding between atoms in a molecule.
(b) Displace the total number of lone pairs representing each of the Atoms of molecules.
(c) Reflect the electronic structure of elements.
In Lewis dot structure, each dot represents an electron and a pair of dots between chemical symbols for atoms represents a bond.
The molecule formula of ethane is \[{C_2}{H_6}\] and the structure formula of ethane is \[C{H_3} - C{H_3}\] .
There are two carbons present in ethane molecules that are single bonded with each other and three hydrogen atoms are present with each carbon.
The electron dot structure of ethane is

Note: Electron-dot structure also known as Lewis start structure actually shows the nature of bond and the position of atoms of the molecule that are connected in the molecule.
It is important and to be noted that only the valence electrons are considered while drawing the Lewis dot structures and the other electrons that do not belong to the outermost shell are ignored.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE

Differentiate between calcination and roasting class 11 chemistry CBSE




