
Draw the electron dot structure of – (1+1+1)
a) Propanone
b) Chloro methane
c) Pentyne
Answer
542.1k+ views
Hint: The electron dot structure which is also called as the Lewis dot structure is the representation of valence electrons of the atom around its elemental symbol in the molecule. Electron dot structure mainly shows how an atom in the molecule makes a bond by sharing the electrons.
Complete answer:
From your chemistry lessons you have learned about the electron dot structure of a molecule. Electron dot is defined as the representation of the molecule it shows the bonding between the atoms of a molecule and it represents the electrons and the lone pair presents on the atom. Electron dot structure is also called the Lewis dot structures or Lewis dot diagram. The dot word that we have used in the naming represents the electrons present in a molecule and the electrons are written as a dot in the diagram.
So, to draw the electron dot structure for any molecule there are following steps that are:
1) Firstly to draw the electron dot structure we have to find the number of valence electrons of each and every atom of a molecule.
2) Then we have to find the number of electrons that is required by each and every atom of the molecule to complete its octet.
3) Then the third step is to find the number of bonds that is included in the molecule.
4) Then we have to draw the skeletal structure of a molecule
5) Lastly we have to place the electrons outside the atoms.
Let us draw the electron dot structure for each of them :
a)Propanone $(C{{H}_{3}}-CO-C{{H}_{3}})$
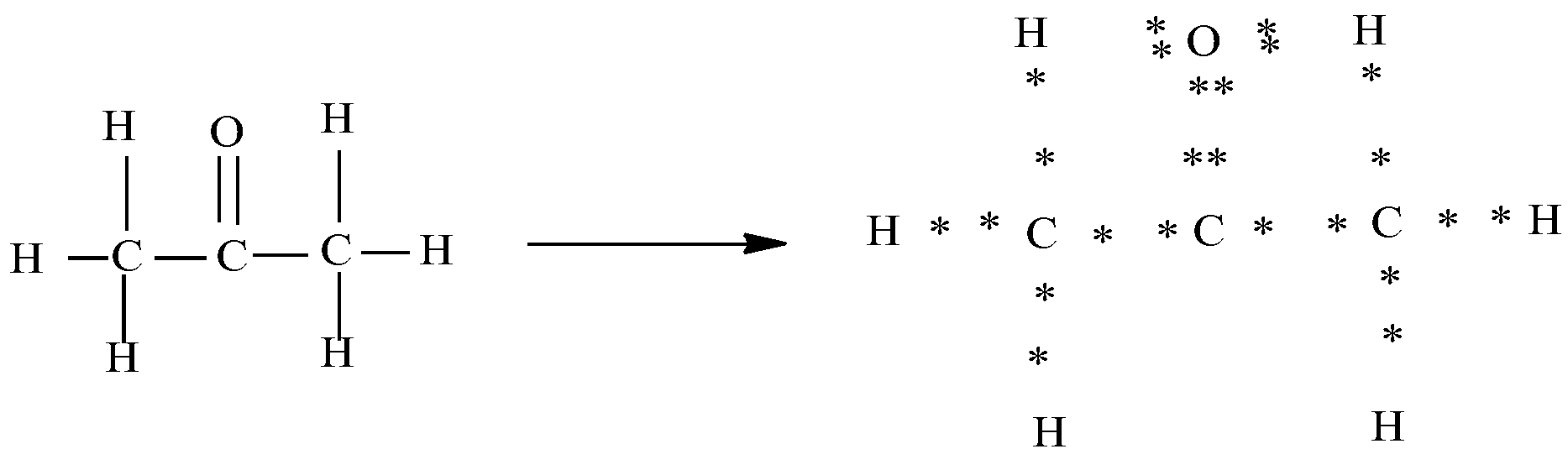
In the above diagram you can see that each of the bonds is represented as the sharing of the electrons. Here the dots present around the oxygen and carbon represent its valence electrons. Oxygen has six valence electrons and carbon has four valence electrons . In the electron dot structure we can see that carbon shares two of its electrons with oxygen and one electron each with two other carbon.
b) Chloro methane $({{H}_{3}}C-Cl)$
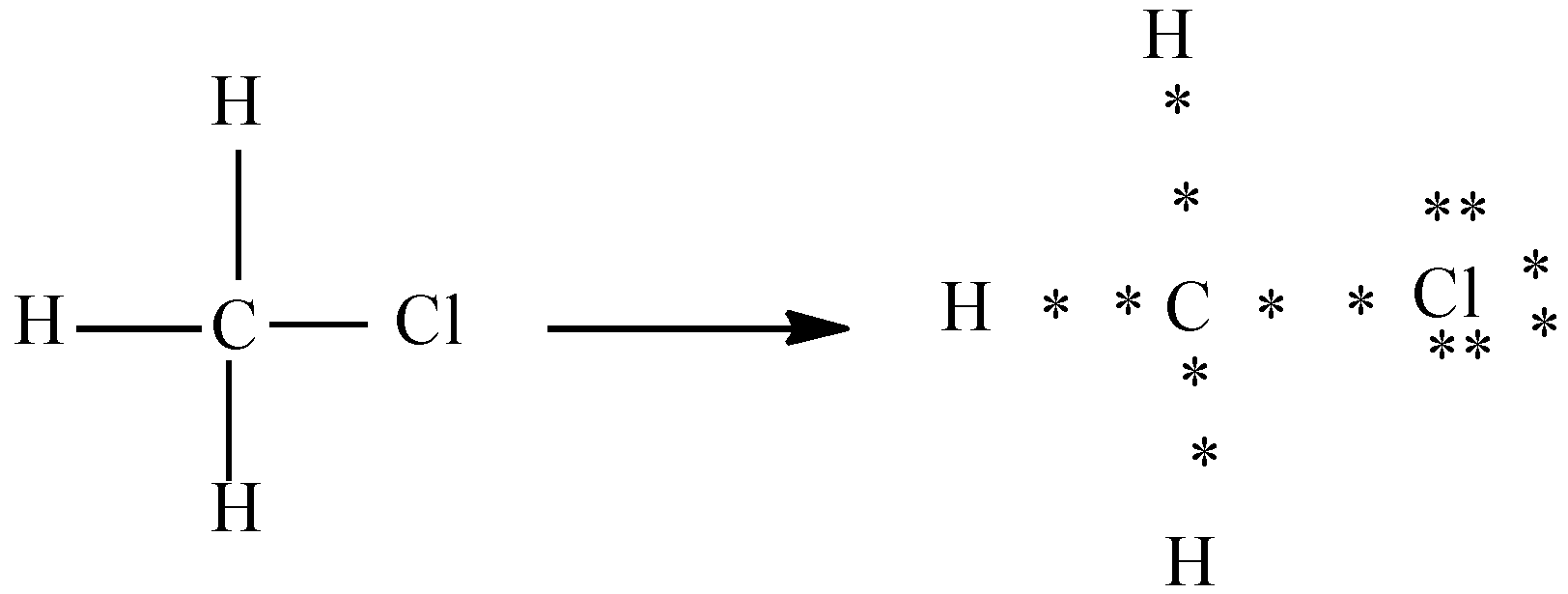
In the above diagram chlorine has 7 valence electrons and carbon has 4 valence electrons. Here we can see that out of seven electrons chlorine shares one electron with carbon and forms the bond and the other electrons are represented as lone pairs.
c) Pentyne $(C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C\equiv CH)$
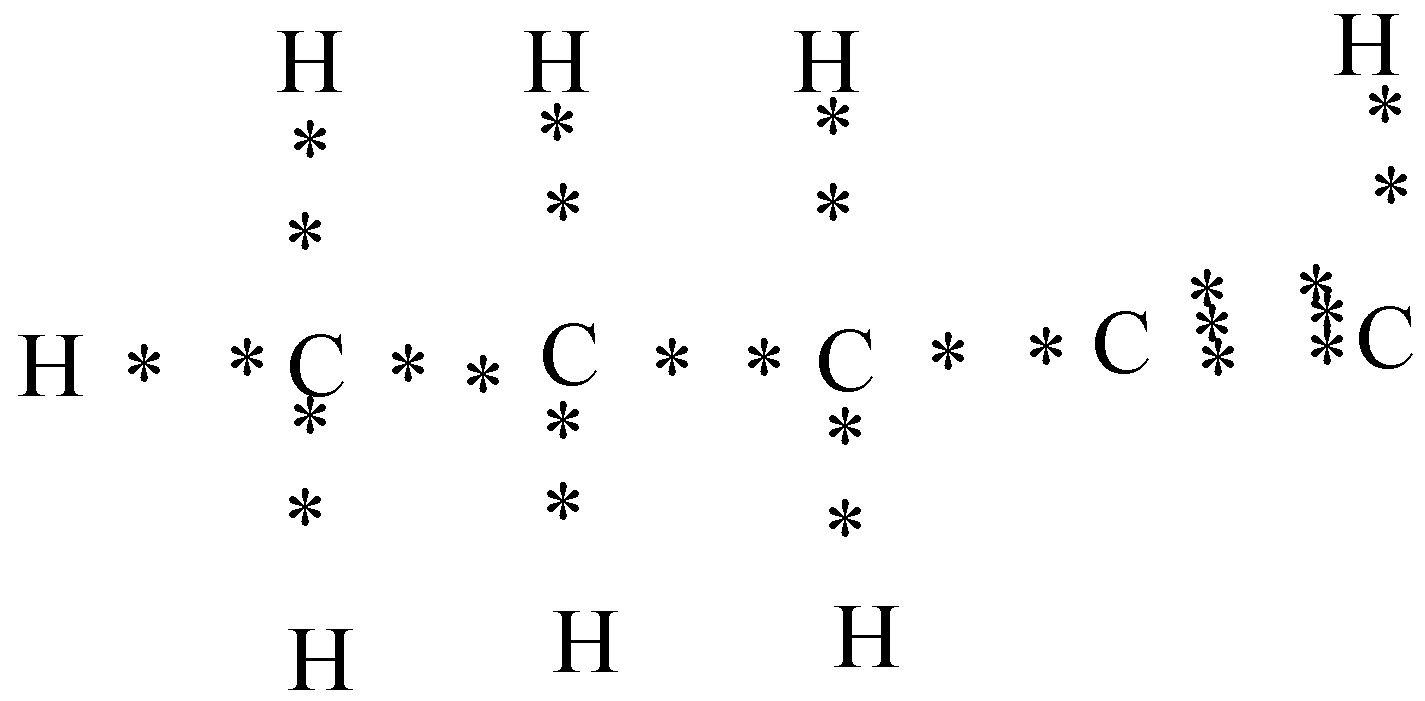
In the above diagram you can see that four valence electrons are present in carbon and to form the pentyne two carbon atoms share their three electrons with each other to form the triple bond.
Note:
From the above diagrams you can see that the maximum number of the common electrons that are present on the adjacent atom of the molecule takes part in sharing and bonding. In case if an element needs only one electron to complete its octet then the number of electrons that are present in the valence shell does not matter.
Complete answer:
From your chemistry lessons you have learned about the electron dot structure of a molecule. Electron dot is defined as the representation of the molecule it shows the bonding between the atoms of a molecule and it represents the electrons and the lone pair presents on the atom. Electron dot structure is also called the Lewis dot structures or Lewis dot diagram. The dot word that we have used in the naming represents the electrons present in a molecule and the electrons are written as a dot in the diagram.
So, to draw the electron dot structure for any molecule there are following steps that are:
1) Firstly to draw the electron dot structure we have to find the number of valence electrons of each and every atom of a molecule.
2) Then we have to find the number of electrons that is required by each and every atom of the molecule to complete its octet.
3) Then the third step is to find the number of bonds that is included in the molecule.
4) Then we have to draw the skeletal structure of a molecule
5) Lastly we have to place the electrons outside the atoms.
Let us draw the electron dot structure for each of them :
a)Propanone $(C{{H}_{3}}-CO-C{{H}_{3}})$

In the above diagram you can see that each of the bonds is represented as the sharing of the electrons. Here the dots present around the oxygen and carbon represent its valence electrons. Oxygen has six valence electrons and carbon has four valence electrons . In the electron dot structure we can see that carbon shares two of its electrons with oxygen and one electron each with two other carbon.
b) Chloro methane $({{H}_{3}}C-Cl)$

In the above diagram chlorine has 7 valence electrons and carbon has 4 valence electrons. Here we can see that out of seven electrons chlorine shares one electron with carbon and forms the bond and the other electrons are represented as lone pairs.
c) Pentyne $(C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C\equiv CH)$

In the above diagram you can see that four valence electrons are present in carbon and to form the pentyne two carbon atoms share their three electrons with each other to form the triple bond.
Note:
From the above diagrams you can see that the maximum number of the common electrons that are present on the adjacent atom of the molecule takes part in sharing and bonding. In case if an element needs only one electron to complete its octet then the number of electrons that are present in the valence shell does not matter.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




