
How do you draw the cis and trans isomers for 1-bromo-4-chlorocyclohexane?
Answer
563.7k+ views
Hint: Isomers are compounds with the same molecular formula but the different structural formula and different properties/arrangements in space. Isomerism is the process of holding isomers and it can be classified into different types as structural, functional, stereo, and geometrical isomerism based on its structure, arrangement, and properties. The well-known example of isomers is ether and alcohol which are functional isomers with each other. Cis and Trans are the isomers based on the position of which substituents get added.
Complete step by step answer:
cis isomers are the compound in which the substituents are facing towards the same side i.e. substituents are present either front side or backside of the atom attached. The cis structure for 1-bromo-4-chlorocyclohexane can be drawn as,
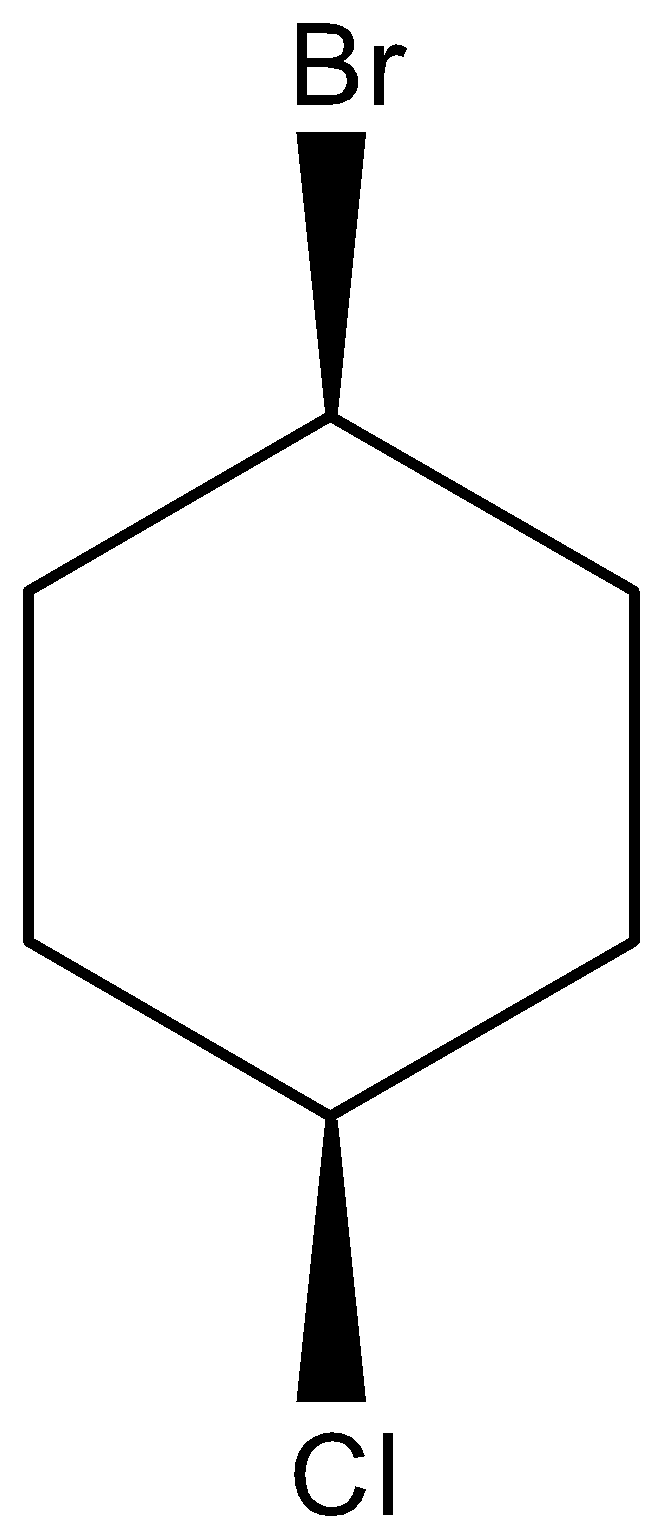
Similarly, trans isomers are the compound in which the substituents are facing opposite to each other. i.e. if one substituent is present at the front side of the atom attached, then the other substituent will be present on the backside of the atom attached. The trans structure for 1-bromo-4-chlorocyclohexane can be drawn as,
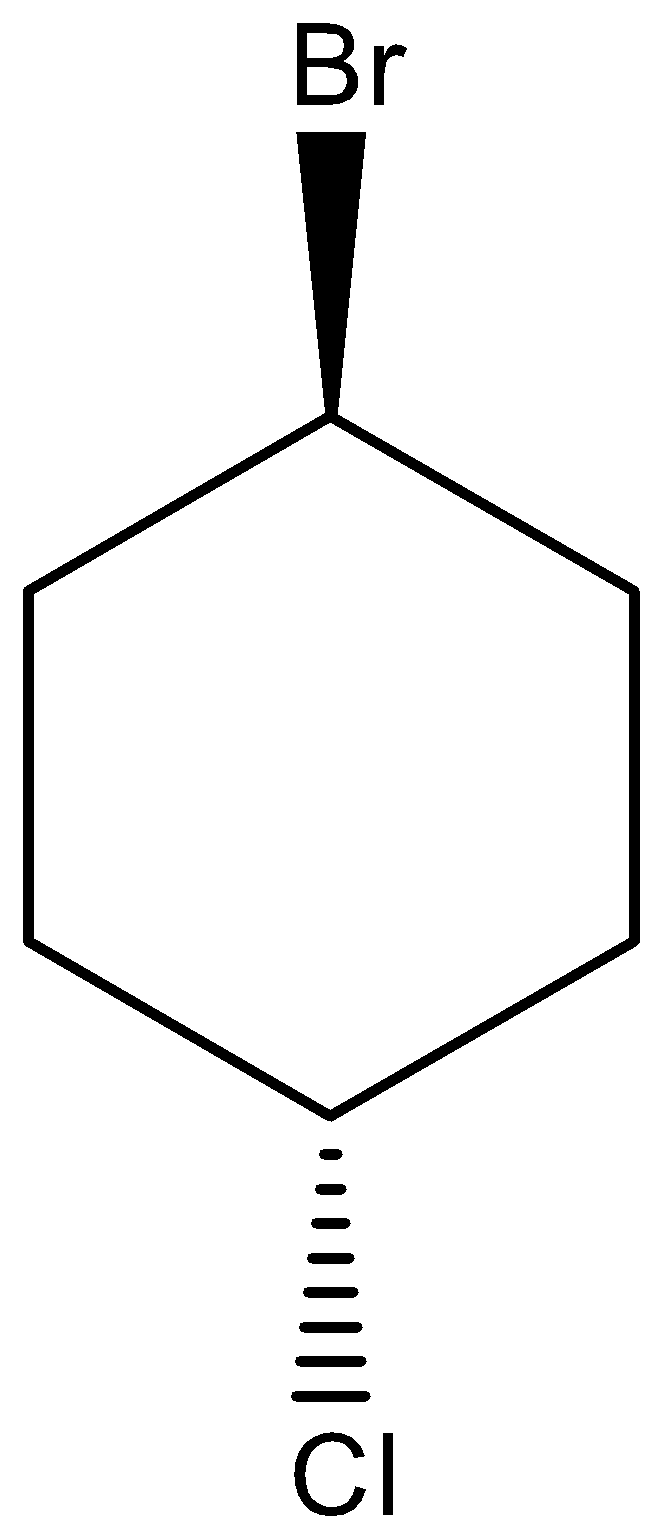
Note: Structural isomers are the compound with the same molecular formula but different structural formula. Under structural isomerism, functional, chain, and tautomer are present. Stereoisomers are the compound with the same molecular formula but different orientation in space. Under stereoisomers, geometry and optical isomers are present. Usually, in acyclic compounds, trans isomers are most stable than cis isomers due to the absence of steric effect in Trans isomers. But, in cyclic compounds, cis cycloalkanes are more stable than trans cycloalkanes since trans cycloalkanes show high ring strain than cis cycloalkanes.
Complete step by step answer:
cis isomers are the compound in which the substituents are facing towards the same side i.e. substituents are present either front side or backside of the atom attached. The cis structure for 1-bromo-4-chlorocyclohexane can be drawn as,

Similarly, trans isomers are the compound in which the substituents are facing opposite to each other. i.e. if one substituent is present at the front side of the atom attached, then the other substituent will be present on the backside of the atom attached. The trans structure for 1-bromo-4-chlorocyclohexane can be drawn as,

Note: Structural isomers are the compound with the same molecular formula but different structural formula. Under structural isomerism, functional, chain, and tautomer are present. Stereoisomers are the compound with the same molecular formula but different orientation in space. Under stereoisomers, geometry and optical isomers are present. Usually, in acyclic compounds, trans isomers are most stable than cis isomers due to the absence of steric effect in Trans isomers. But, in cyclic compounds, cis cycloalkanes are more stable than trans cycloalkanes since trans cycloalkanes show high ring strain than cis cycloalkanes.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

Which out of the following hydrocarbons undergo addition class 11 chemistry CBSE




