
Draw structure of $HCl{O_4}$ .
Answer
583.5k+ views
Hint: In order to solve the given problem we will study in brief about the given compound and also the different types of bonds present in the structure of the compound further on the basis of the discussion we will draw the structure of the given compound. Also we will see the different characteristics and the uses of the given compound.
Complete step by step answer:
Let us first study in brief about the given compound.
The given compound $HCl{O_4}$ is an acid and its name is Perchloric acid.
$HCl{O_4}$ is an oxoacid containing chlorine with the chemical name Perchloric Acid. Hyperchloric acid $HCl{O_4}$ or hydroxide trioxido chlorine are also identified. A pure odourless colourless aqueous solution is between 50 percent to 72 percent acid. Tissue and metals are corrosive to it. It will rupture violently when closed containers are subjected to heat for a long time.
It can be assembled at a global level in two ways. The high aqueous solubility of sodium perchlorate is thoroughly used in the conventional process. Perchloric acid is formed by the precipitation of solid sodium chloride by treating this solution with hydrochloric acid.
The structure of the Perchloric acid is:
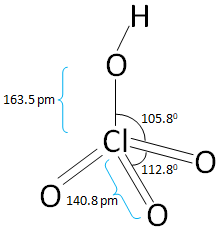
In the above figure of Perchloric acid all the bond length and the bond angles are mentioned.
Now let us see some of the uses of Perchloric acid.
1. Perchloric acid is used in the processing of sodium and potassium as an oxidiser.
2. Perchloric acid used in explosives manufacturing.
3. Perchloric acid used with decorative plating.
4. Perchloric acid used for 1H-Benzotriazole determination as a reagent
5. Perchloric acid utilized as a catalyst.
6. Perchloric acid used in rocket fueling.
7. Perchloric acid used in molybdenum electropolishing.
Note: In order to solve such types of problems related to the diagram of the compound students must remember the different types of bonds that exists between the atoms of different in a compound and should also remember the basic bond length and bond angles for single double bond and different types of geometrical like tetrahedral, octahedral etc.
Complete step by step answer:
Let us first study in brief about the given compound.
The given compound $HCl{O_4}$ is an acid and its name is Perchloric acid.
$HCl{O_4}$ is an oxoacid containing chlorine with the chemical name Perchloric Acid. Hyperchloric acid $HCl{O_4}$ or hydroxide trioxido chlorine are also identified. A pure odourless colourless aqueous solution is between 50 percent to 72 percent acid. Tissue and metals are corrosive to it. It will rupture violently when closed containers are subjected to heat for a long time.
It can be assembled at a global level in two ways. The high aqueous solubility of sodium perchlorate is thoroughly used in the conventional process. Perchloric acid is formed by the precipitation of solid sodium chloride by treating this solution with hydrochloric acid.
The structure of the Perchloric acid is:

In the above figure of Perchloric acid all the bond length and the bond angles are mentioned.
Now let us see some of the uses of Perchloric acid.
1. Perchloric acid is used in the processing of sodium and potassium as an oxidiser.
2. Perchloric acid used in explosives manufacturing.
3. Perchloric acid used with decorative plating.
4. Perchloric acid used for 1H-Benzotriazole determination as a reagent
5. Perchloric acid utilized as a catalyst.
6. Perchloric acid used in rocket fueling.
7. Perchloric acid used in molybdenum electropolishing.
Note: In order to solve such types of problems related to the diagram of the compound students must remember the different types of bonds that exists between the atoms of different in a compound and should also remember the basic bond length and bond angles for single double bond and different types of geometrical like tetrahedral, octahedral etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




