
How can I draw possible structures for an ester that has a molecular ion with a m/z value of 74.
Answer
547.2k+ views
Hint: In mass spectroscopy, the molecular ion peak will give the information of the molecular weight of a particular compound. Molecular ion peaks are sometimes also called base peaks. By using molecular ion peaks we can find the structure of a particular compound.
Complete answer:
- In the question is asked the draw the possible structures for an ester that has a molecular ion with m/z values is 74.
- Molecular ion m/z 74 is nothing but molecular ion peak is at 74. Means 74 is the molecular weight of the particular compound.
- In the question it is given that the molecule contains a ester functional group.
- The molecular formula to represent an ester functional group is RCOOR.
- The molecular weight of the ‘COO’ ester is 44 (12+16+16).
- Means now we have to find the groups or atoms whose molecular weight is 74- 44 = 30.
- The chemical formula to represent an alkane is ${{C}_{n}}{{H}_{2n+2}}$ .
- The molecular weight of the carbon is 12 and the molecular weight of the hydrogen is ‘1’.
- Now we can calculate the value of ‘n’ in the above formula as follows.
12n+1(2n+2) = 14n+2 = 30
n = 2.
- n = 2 means there are two methyl groups (two carbons more) are there in the ester compound in place of ‘R’.
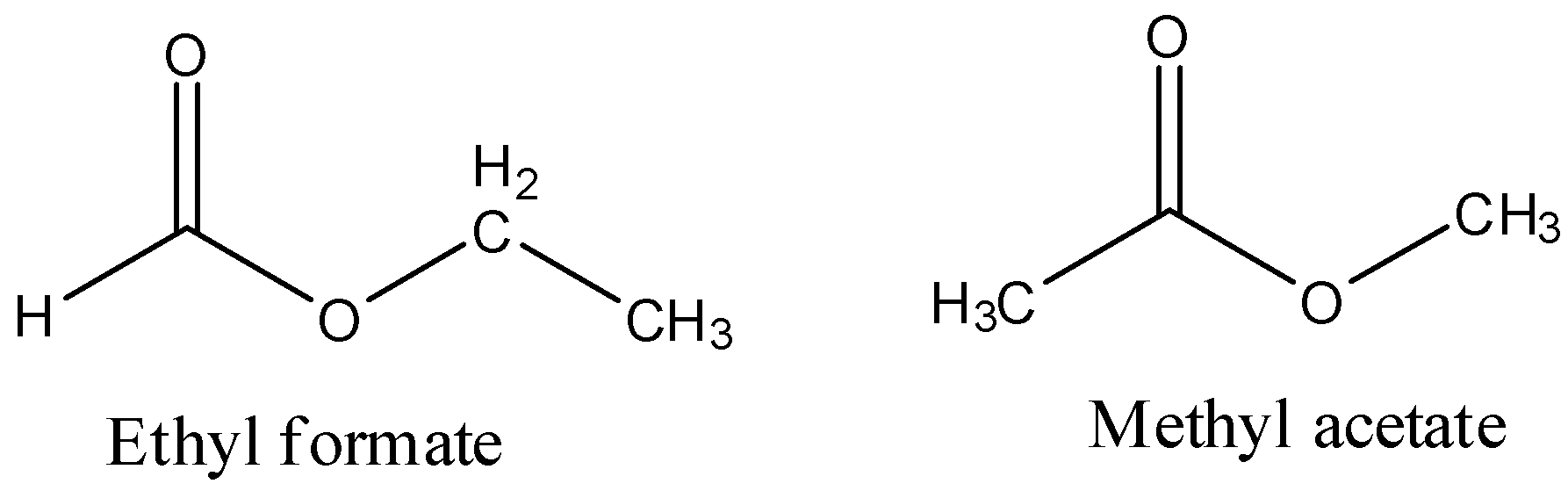
- We can write the possible two structures of the esters with a molecular weight 74 and they are as follows.
Note:
The importance of mass spectroscopy is we can find the molecular weight of the organic compound very easily. By using the molecular weight from the molecular peak ion peak we can find the structure of the molecule based on proton NMR data.
Complete answer:
- In the question is asked the draw the possible structures for an ester that has a molecular ion with m/z values is 74.
- Molecular ion m/z 74 is nothing but molecular ion peak is at 74. Means 74 is the molecular weight of the particular compound.
- In the question it is given that the molecule contains a ester functional group.
- The molecular formula to represent an ester functional group is RCOOR.
- The molecular weight of the ‘COO’ ester is 44 (12+16+16).
- Means now we have to find the groups or atoms whose molecular weight is 74- 44 = 30.
- The chemical formula to represent an alkane is ${{C}_{n}}{{H}_{2n+2}}$ .
- The molecular weight of the carbon is 12 and the molecular weight of the hydrogen is ‘1’.
- Now we can calculate the value of ‘n’ in the above formula as follows.
12n+1(2n+2) = 14n+2 = 30
n = 2.
- n = 2 means there are two methyl groups (two carbons more) are there in the ester compound in place of ‘R’.
- We can write the possible two structures of the esters with a molecular weight 74 and they are as follows.

Note:
The importance of mass spectroscopy is we can find the molecular weight of the organic compound very easily. By using the molecular weight from the molecular peak ion peak we can find the structure of the molecule based on proton NMR data.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




