
Draw orbit structure diagram of Calcium oxide \[\left( {CaO} \right)\].
Answer
566.7k+ views
Hint:In the given question firstly we have to define the facts regarding the polarity and the no polarity and the basic definition. Calcium oxide (CaO), its other names are quicklime or burnt lime. It is a widely used chemical compound in industrial areas. It is white in colour, , alkaline in nature and crystalline solid at room temperature.
Complete step-by-step answer:The given question statement asks about the structure of the compound of calcium oxide, the integrative structure of the elements as a self and in the compound as a molecule.
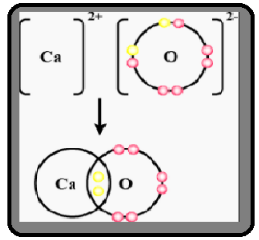
Calcium oxide (CaO), is also known as quicklime or burnt lime. It is a widely used chemical compound. It is a white in colour , alkaline in nature, crystalline solid at room temperature. The broadly used term "lime" denotes calcium-containing inorganic materials, in which carbonates, oxides and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. The name quicklime specifically used for only one chemical compound calcium oxide. Calcium oxide that survives processing without reacting in building products such as cement is called free lime.] Calcium oxide is made up by heating limestone ( $CaC{O_3}$ ). On heating $C{O_2}$ gas evolves and $CaO$ is formed
$CaC{O_3} \to CaO + C{O_2}$
Quicklime is relatively inexpensive. $CaO$ and its chemical derivative (calcium hydroxide, of which quicklime is the anhydride) are important commodity chemicals.
Note:Quicklime and hydrated lime is used to increase the load carrying capacity of clay-containing soils. They do this by reacting finely with divided silica and alumina to produce calcium silicates and aluminates, which possess cementing properties.
Complete step-by-step answer:The given question statement asks about the structure of the compound of calcium oxide, the integrative structure of the elements as a self and in the compound as a molecule.

Calcium oxide (CaO), is also known as quicklime or burnt lime. It is a widely used chemical compound. It is a white in colour , alkaline in nature, crystalline solid at room temperature. The broadly used term "lime" denotes calcium-containing inorganic materials, in which carbonates, oxides and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. The name quicklime specifically used for only one chemical compound calcium oxide. Calcium oxide that survives processing without reacting in building products such as cement is called free lime.] Calcium oxide is made up by heating limestone ( $CaC{O_3}$ ). On heating $C{O_2}$ gas evolves and $CaO$ is formed
$CaC{O_3} \to CaO + C{O_2}$
Quicklime is relatively inexpensive. $CaO$ and its chemical derivative (calcium hydroxide, of which quicklime is the anhydride) are important commodity chemicals.
Note:Quicklime and hydrated lime is used to increase the load carrying capacity of clay-containing soils. They do this by reacting finely with divided silica and alumina to produce calcium silicates and aluminates, which possess cementing properties.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




