
How can I draw for cis-1,2-diethylcyclohexane the two chair conformers and indicate which conformer is more stable?
Answer
547.2k+ views
Hint: First we should know about the axial and equatorial positions in the chair form of cyclohexane to draw the cis-1,2-diethylcyclohexane in chair form. The structure which represents the equatorial and axial positions in cyclohexane is as follows.
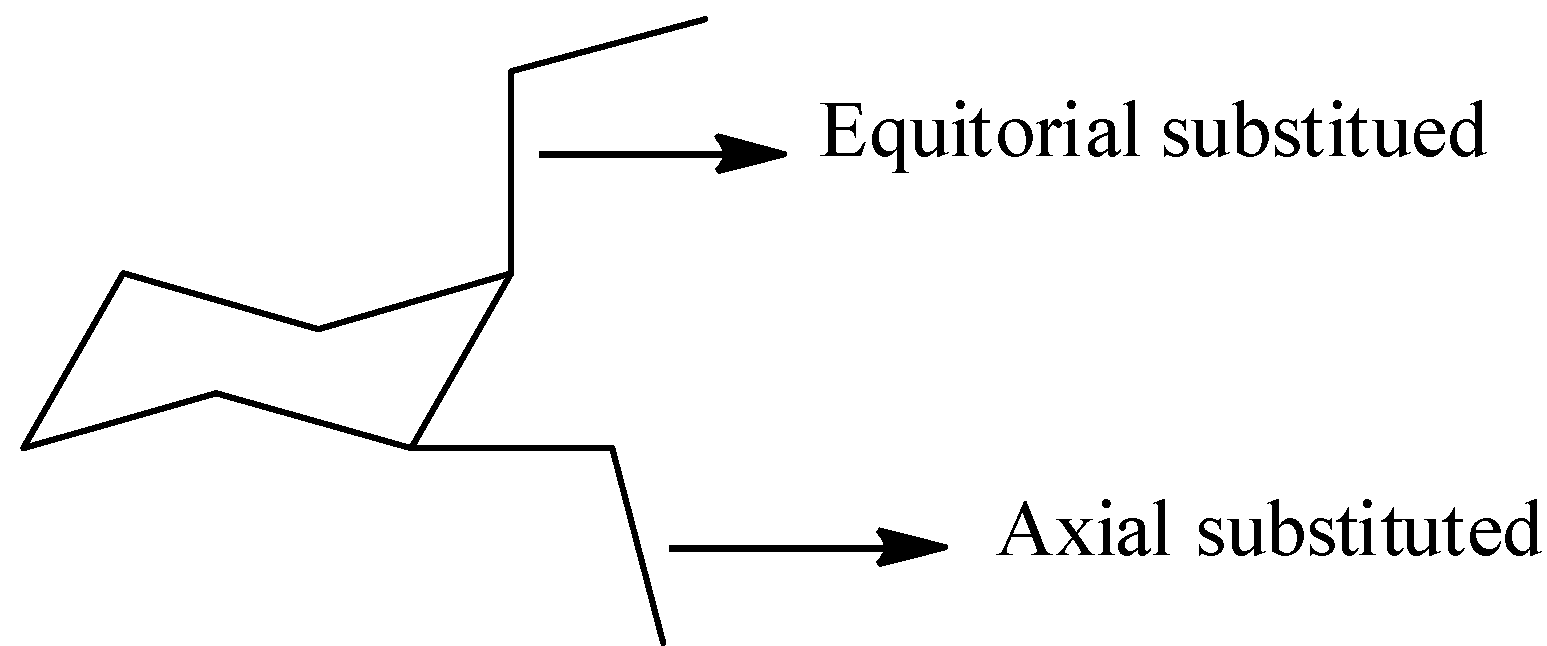
Complete answer:
- In the question it is asked to draw the two chair conformers of cis-1,2-diethylcyclohexane and indicate which conformer is more stable.
- In the question the given molecule is cis-1,2-diethylcyclohexane, means two ethyl groups are cis to each other in chair conformers.
- The possible two chair conformers of the given compound, cis-1,2-diethylcyclohexane is as follows.
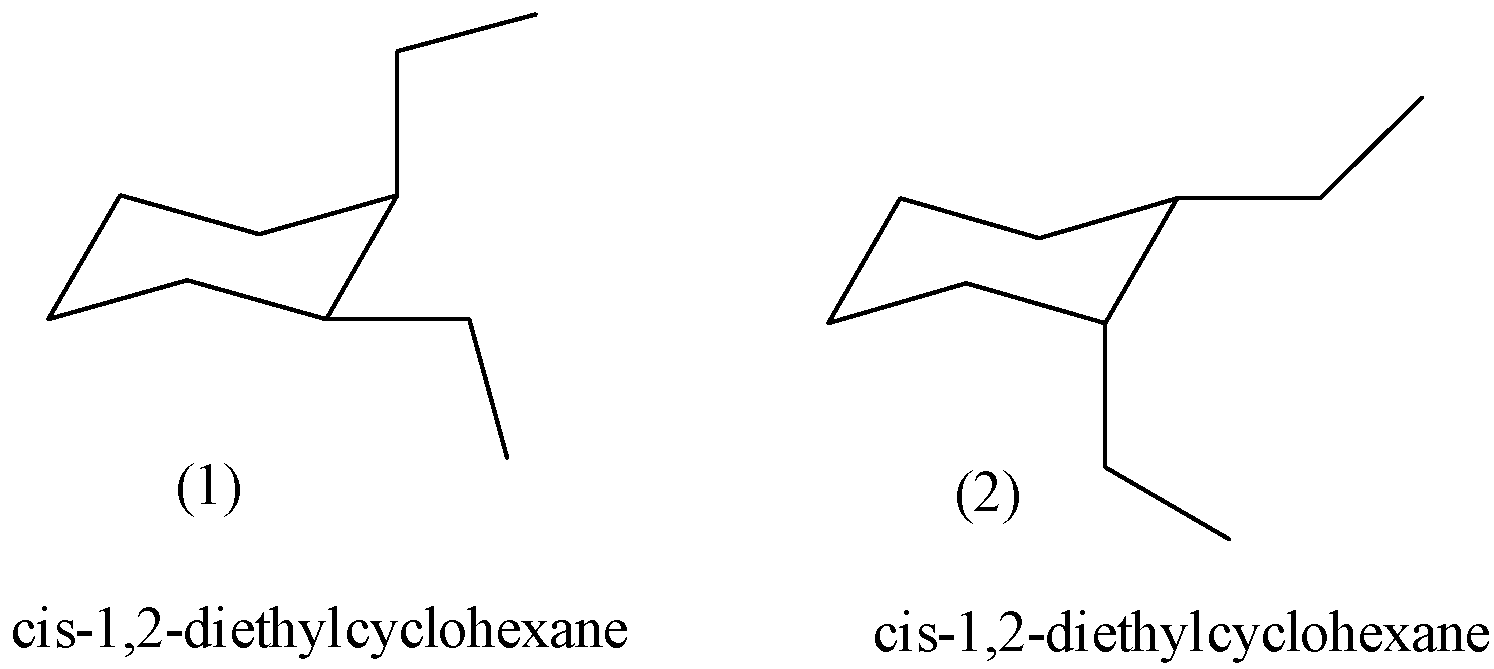
- The above two structures are going to represent the same molecule cis-1,2-diethylcyclohexane.
- In structure- 1, one ethyl group is at equatorial position and other ethyl group at axial position to become cis to each other.
- In structure- 2, one ethyl group is at axial position and other ethyl group at equitorail position to become cis to each other.
- In both the structures the amount of energy going to carry is same by the both the possible chair conformers of cis-1,2-diethylcyclohexane.
- Therefore both the chair conformers are equally stable in nature.
- If one chair conformer is cis and the other is trans then trans chair conformer is more stable than the cis chair conformer.
Note:
In case of chair conformer trans chair conformers are more stable than the cis chair conformers due to the presence of less repulsions in case of trans chair conformer when compared to cis chair conformer.

Complete answer:
- In the question it is asked to draw the two chair conformers of cis-1,2-diethylcyclohexane and indicate which conformer is more stable.
- In the question the given molecule is cis-1,2-diethylcyclohexane, means two ethyl groups are cis to each other in chair conformers.
- The possible two chair conformers of the given compound, cis-1,2-diethylcyclohexane is as follows.

- The above two structures are going to represent the same molecule cis-1,2-diethylcyclohexane.
- In structure- 1, one ethyl group is at equatorial position and other ethyl group at axial position to become cis to each other.
- In structure- 2, one ethyl group is at axial position and other ethyl group at equitorail position to become cis to each other.
- In both the structures the amount of energy going to carry is same by the both the possible chair conformers of cis-1,2-diethylcyclohexane.
- Therefore both the chair conformers are equally stable in nature.
- If one chair conformer is cis and the other is trans then trans chair conformer is more stable than the cis chair conformer.
Note:
In case of chair conformer trans chair conformers are more stable than the cis chair conformers due to the presence of less repulsions in case of trans chair conformer when compared to cis chair conformer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




