
Draw electron dot structure of Butane.
Answer
503.4k+ views
Hint: Electron dot structure is also known as Lewis dot structure. Lewis dot structure can be made of covalent compounds. To draw the electron dot structure of any compound, we should know its formula. In this type of structure, electrons are denoted with dots and only valence electrons (electrons in the outermost shell of the atom) are shown in the structure.
Complete answer:
We know that electron dot structure is known as Lewis dot structure. In this structure, only valence electrons of an atom are shown. Valence electrons are those electrons which are present in the outermost shell of an electron.
To draw the electron dot structure for any compound, we first need to know its chemical formula. So, butane is an alkane with a chain of four carbons. Its chemical formula is ${C_4}{H_{10}}.$
All the bonds present in a butane molecule are covalent bonds which means that the electrons are shared between the carbon and hydrogen. Electron dot structure of butane is given as:
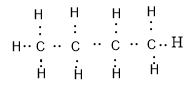
We can see that there are four valence electrons of carbon and one valence electron of hydrogen because the electronic configurations of carbon and hydrogen are:
$C = 1{s^2}2{s^2}2{p^2}$
$H = 1{s^1}$
Note:
Butane has a faint petroleum-like odour and it is a colourless gas. It has many applications like it can be used as a food propellant and also as a refrigerant. It is a highly flammable and easily liquefiable gas. Butane is a saturated hydrocarbon as it does not contain any multiple bonds in its structure.
Complete answer:
We know that electron dot structure is known as Lewis dot structure. In this structure, only valence electrons of an atom are shown. Valence electrons are those electrons which are present in the outermost shell of an electron.
To draw the electron dot structure for any compound, we first need to know its chemical formula. So, butane is an alkane with a chain of four carbons. Its chemical formula is ${C_4}{H_{10}}.$
All the bonds present in a butane molecule are covalent bonds which means that the electrons are shared between the carbon and hydrogen. Electron dot structure of butane is given as:
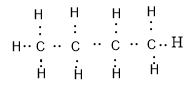
We can see that there are four valence electrons of carbon and one valence electron of hydrogen because the electronic configurations of carbon and hydrogen are:
$C = 1{s^2}2{s^2}2{p^2}$
$H = 1{s^1}$
Note:
Butane has a faint petroleum-like odour and it is a colourless gas. It has many applications like it can be used as a food propellant and also as a refrigerant. It is a highly flammable and easily liquefiable gas. Butane is a saturated hydrocarbon as it does not contain any multiple bonds in its structure.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




