
Draw electron dot representation for the formation of magnesium chloride.
Answer
584.7k+ views
Hint: Chemical formula is a way to write a substance using the chemical symbol and number subscripts to mention the number of the atoms into that chemical compound. Magnesium chloride is an inorganic salt. The formula of Magnesium chloride is \[MgC{l_2}\] .
Complete step by step answer:
Magnesium chloride can be prepared from magnesium chloride hydrated using HCl gas.
A Lewis dot representation diagram is a representation of the valence electrons of an atom that uses the dots around the symbol of the element. The number of dots is equal to the valence electron of that atom.
The valence electron of Magnesium is 2. And the valence electron of chlorine is 7. The dot representation of magnesium chloride is,
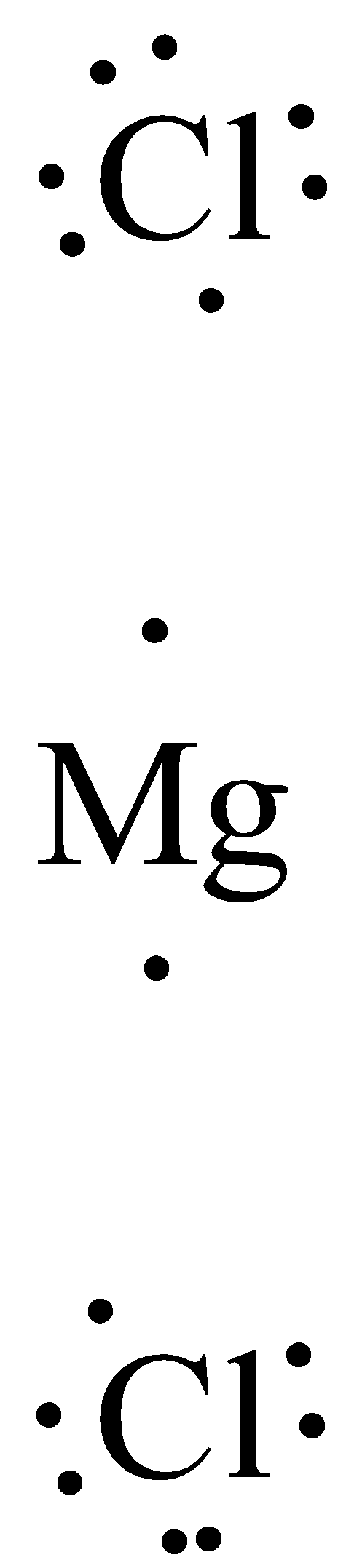
Additional information:
Grignard’s reagent is a metal alkyl halide formed when alkyl halide reacts with metal such as magnesium, for example, RMgX where R can be any alkyl group like methyl, ethyl, etc. They are the first source of carbanions or Lewis bases or electron-pair donors.
We can say that the carbonyl carbon of acetaldehyde has a positive charge and the oxygen has a negative charge. So, when Grignard reagent attacks, it pushes the double bond between oxygen and carbon and leaves a lone pair on oxygen that contain a metal-carbon bond. This produces magnesium halide along with the product.
Acetaldehyde \[(C{H_3}CHO)\] on reacting with Grignard’s reagent \[(C{H_3}MgBr)\] in presence of diethyl ether will now result in a chain of three carbons which on hydrolysis give the main product having secondary alcohol i.e. Propan-2-ol.
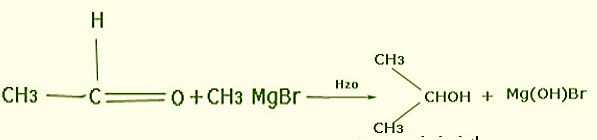
Note:Magnesium is a naturally occurring mineral. It is important for many systems in our body especially in the case of nerves and muscles. Magnesium chloride is used to treat or prevent any magnesium deficiency.
Complete step by step answer:
Magnesium chloride can be prepared from magnesium chloride hydrated using HCl gas.
A Lewis dot representation diagram is a representation of the valence electrons of an atom that uses the dots around the symbol of the element. The number of dots is equal to the valence electron of that atom.
The valence electron of Magnesium is 2. And the valence electron of chlorine is 7. The dot representation of magnesium chloride is,
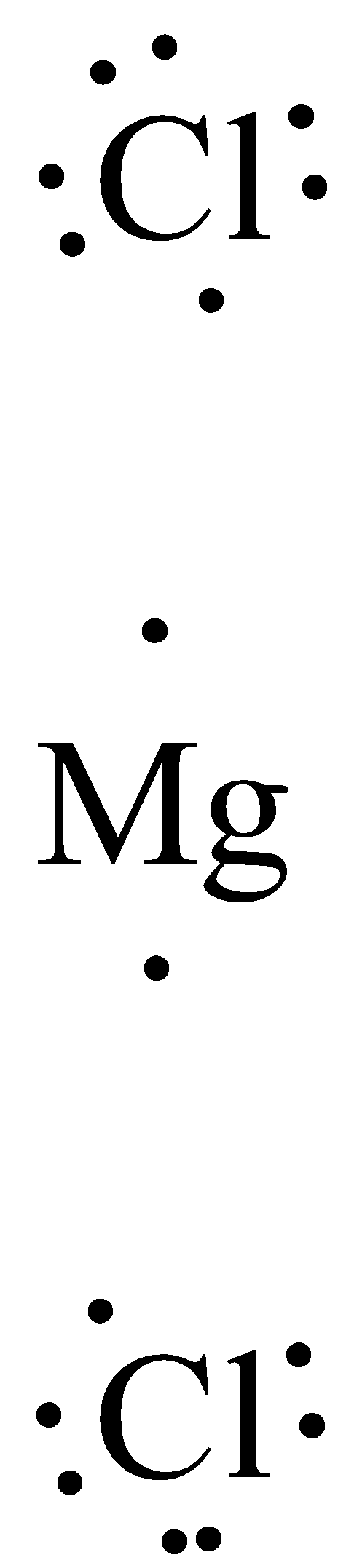
Additional information:
Grignard’s reagent is a metal alkyl halide formed when alkyl halide reacts with metal such as magnesium, for example, RMgX where R can be any alkyl group like methyl, ethyl, etc. They are the first source of carbanions or Lewis bases or electron-pair donors.
We can say that the carbonyl carbon of acetaldehyde has a positive charge and the oxygen has a negative charge. So, when Grignard reagent attacks, it pushes the double bond between oxygen and carbon and leaves a lone pair on oxygen that contain a metal-carbon bond. This produces magnesium halide along with the product.
Acetaldehyde \[(C{H_3}CHO)\] on reacting with Grignard’s reagent \[(C{H_3}MgBr)\] in presence of diethyl ether will now result in a chain of three carbons which on hydrolysis give the main product having secondary alcohol i.e. Propan-2-ol.

Note:Magnesium is a naturally occurring mineral. It is important for many systems in our body especially in the case of nerves and muscles. Magnesium chloride is used to treat or prevent any magnesium deficiency.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




