
How do you draw ${C_8}{H_{18}}$ (octane)? How many isomers are there? What are the names of the isomers?
Answer
566.1k+ views
Hint: From the given molecular formula of octane ${C_8}{H_{18}}$, it can be known that the total number of carbon atom present in octane is 8 and the number of hydrogen atom present in octane is 18.
Complete step by step answer:
The octane is a hydrocarbon with 8 carbon atoms and 18 hydrogen atoms. The molecular formula of octane is ${C_8}{H_{18}}$.
The carbon is a tetravalent atom. It contains 4 electrons in the valence shell so it can donate its four electrons to form bonds with four other atoms.
The structure of octane is drawn by first drawing a straight chain or parent chain of 8 carbons and attaching 18 hydrogen on carbon to fulfill its tetravalency.
The structure of octane is shown below.
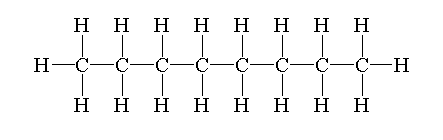
Isomers are defined as the molecules which have similar formulae but have different arrangements of atoms in the space.
Octane has a total 18 isomers.
(1) Octane
(2) 2-Methyl heptane
(3) 3-Methyl heptane
(4) 4-Methyl heptane
(5) 2, 2-Dimethyl hexane
(6) 2, 3-Dimethyl hexane
(7) 2, 4-Dimethyl hexane
(8) 2, 5-Dimethyl hexane
(9) 3, 3-Dimethyl hexane
(10) 3, 4-Dimethyl hexane
(11) 3-Ethyl hexane
(12) 2, 2, 3-Trimethylpentane
(13) 2, 2, 4-Trimethyl pentane
(14) 2, 3, 3-Trimethylpentane
(15) 2, 3, 4-Trimethyl pentane
(16) 2-Methyl-3-ethyl pentane
(17) 3-Methyl-3-ethyl pentane
(18) Tetramethylbutane
Note:
These isomers are also known as structural isomers as the structural representation of the atoms is different in each isomer but the number of atoms are same in each isomer.
Complete step by step answer:
The octane is a hydrocarbon with 8 carbon atoms and 18 hydrogen atoms. The molecular formula of octane is ${C_8}{H_{18}}$.
The carbon is a tetravalent atom. It contains 4 electrons in the valence shell so it can donate its four electrons to form bonds with four other atoms.
The structure of octane is drawn by first drawing a straight chain or parent chain of 8 carbons and attaching 18 hydrogen on carbon to fulfill its tetravalency.
The structure of octane is shown below.

Isomers are defined as the molecules which have similar formulae but have different arrangements of atoms in the space.
Octane has a total 18 isomers.
(1) Octane
(2) 2-Methyl heptane
(3) 3-Methyl heptane
(4) 4-Methyl heptane
(5) 2, 2-Dimethyl hexane
(6) 2, 3-Dimethyl hexane
(7) 2, 4-Dimethyl hexane
(8) 2, 5-Dimethyl hexane
(9) 3, 3-Dimethyl hexane
(10) 3, 4-Dimethyl hexane
(11) 3-Ethyl hexane
(12) 2, 2, 3-Trimethylpentane
(13) 2, 2, 4-Trimethyl pentane
(14) 2, 3, 3-Trimethylpentane
(15) 2, 3, 4-Trimethyl pentane
(16) 2-Methyl-3-ethyl pentane
(17) 3-Methyl-3-ethyl pentane
(18) Tetramethylbutane
Note:
These isomers are also known as structural isomers as the structural representation of the atoms is different in each isomer but the number of atoms are same in each isomer.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




