
Draw an energy level diagram for the reaction:
\[NaOH + HCl \to NaCl + {H_2}O\]
$\Delta H = - 57KJ/mol$
Answer
579.9k+ views
Hint: The energy level diagram is a graph of energy levels of the reaction v/s the progress of the reaction. It gives the information about the energy levels at various stages of the conversion of reactants into products.
Complete step by step answer:
An energy level is a graphical representation of the energy levels of reactants products and intermediates. Before going into the details of the given reaction, few terms need to be understood.
Heat of a reaction is the measurement of the total energy change during a reaction. It can be released or absorbed. It is indicated in the form of positive and negative signs. It is equal to the total energy of a substance. The total energy is the sum of kinetic energy and potential energy of a molecule.
Temperature of a substance is a measure of the initial and final kinetic energies of a substance. It is measured in Kelvin.
In order to plot the diagram for the given reaction, first the heat change of the reaction has to be determined. If heat is released then the reaction is termed exothermic reaction. If heat is absorbed then the reaction is termed endothermic reaction.
In exothermic reactions energy is released due to chemical change and transferred from the reaction medium into the surroundings. As the average kinetic energy of the surroundings increases, so the temperature of reaction mixture increases and it feels warmer. The burning (combustion) of a substance, neutralization reactions of acids and alkalis, dissolution of alkali hydroxides in water are common examples of exothermic reactions.
The given reaction is a type of neutralization reaction between an acid and an alkali. \[NaOH\] is an alkali and \[HCl\] is an acid. The reactants undergo neutralization to produce\[NaCl\], water and heat. Thus it is an exothermic reaction. The value of heat change of the reaction is negative which also confirms that heat is released during the reaction progress.
The energy level diagram for the reaction of \[NaOH + HCl \to NaCl + {H_2}O\] is shown in figure \[1\].
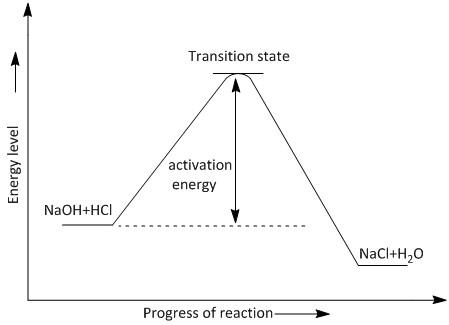
Figure\[1\] : The plot of reaction of \[NaOH\] and\[HCl\] .
The diagram indicates that the energy of products is less than the energy of reactants. The value of negative $\Delta H$ indicates that energy is released and the reaction is an exothermic reaction.
Note: Unlike exothermic reaction, in endothermic reaction energy is absorbed from the surroundings. This results in a decrease of the average kinetic energy of the surroundings thereby the reaction mixture gets colder. The reaction of dissolution of ammonium hydroxide in water is an example of endothermic reaction.
Complete step by step answer:
An energy level is a graphical representation of the energy levels of reactants products and intermediates. Before going into the details of the given reaction, few terms need to be understood.
Heat of a reaction is the measurement of the total energy change during a reaction. It can be released or absorbed. It is indicated in the form of positive and negative signs. It is equal to the total energy of a substance. The total energy is the sum of kinetic energy and potential energy of a molecule.
Temperature of a substance is a measure of the initial and final kinetic energies of a substance. It is measured in Kelvin.
In order to plot the diagram for the given reaction, first the heat change of the reaction has to be determined. If heat is released then the reaction is termed exothermic reaction. If heat is absorbed then the reaction is termed endothermic reaction.
In exothermic reactions energy is released due to chemical change and transferred from the reaction medium into the surroundings. As the average kinetic energy of the surroundings increases, so the temperature of reaction mixture increases and it feels warmer. The burning (combustion) of a substance, neutralization reactions of acids and alkalis, dissolution of alkali hydroxides in water are common examples of exothermic reactions.
The given reaction is a type of neutralization reaction between an acid and an alkali. \[NaOH\] is an alkali and \[HCl\] is an acid. The reactants undergo neutralization to produce\[NaCl\], water and heat. Thus it is an exothermic reaction. The value of heat change of the reaction is negative which also confirms that heat is released during the reaction progress.
The energy level diagram for the reaction of \[NaOH + HCl \to NaCl + {H_2}O\] is shown in figure \[1\].

Figure\[1\] : The plot of reaction of \[NaOH\] and\[HCl\] .
The diagram indicates that the energy of products is less than the energy of reactants. The value of negative $\Delta H$ indicates that energy is released and the reaction is an exothermic reaction.
Note: Unlike exothermic reaction, in endothermic reaction energy is absorbed from the surroundings. This results in a decrease of the average kinetic energy of the surroundings thereby the reaction mixture gets colder. The reaction of dissolution of ammonium hydroxide in water is an example of endothermic reaction.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




