
How do you draw all the stereoisomers of $1 - bromo - 2 - chlorocyclohexane\,?$
Answer
557.1k+ views
Hint: First draw the structure of the compound using IUPAC Nomenclature. Then try to identify the number of chiral centers present in the given molecule. Then by using the formula total number of stereoisomers for a given molecule $ = \,{2^n}$, where $n$ is the number of chiral centers, find out the number the number of stereoisomers and try to draw the structures.
Complete step-by-step answer:The given molecule is $1 - bromo - 2 - chlorocyclohexane$ which can be represented as,
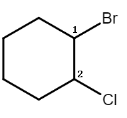
Now we know that for a given molecule the total number of stereoisomers $ = \,{2^n}$, where $n$ is the number of chiral centers.
From the structure we can see there are only two chiral centers present in the molecule, the two chiral centers being ${C_1}$ and ${C_2}$.
So using the formula we get,
Total number of stereoisomers for $1 - bromo - 2 - chlorocyclohexane$ $ = \,\,{2^2}\,\, = \,\,4$.
So there are in total four stereoisomers possible for the given compound, in which in one stereoisomer both the substituents are present above the plane $($denoted by wedge$)$ while in another both the substituents are present below the plane$($denoted by dash$)$, while in other two either one of them is present below the plane and the other above the plane. So the structures of all the possible stereoisomers are:
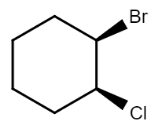
$\left( {1R,2S} \right) - 1 - bromo - 2 - chlorocyclohexane$
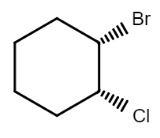
$\left( {1S,2R} \right) - 1 - bromo - 2 - chlorocyclohexane$
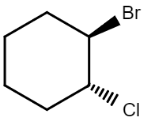
$\left( {1R,2R} \right) - 1 - bromo - 2 - chlorocyclohexane$
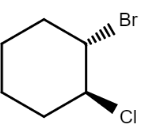
$\left( {1S,2S} \right) - 1 - bromo - 2 - chlorocyclohexane$
Additional Information:Among the four stereoisomers, $\left( {1R,2S} \right) - 1 - bromo - 2 - chlorocyclohexane$ and $\left( {1S,2R} \right) - 1 - bromo - 2 - chlorocyclohexane$ are a pair of enantiomers while $\left( {1R,2R} \right) - 1 - bromo - 2 - chlorocyclohexane$ and $\left( {1S,2S} \right) - 1 - bromo - 2 - chlorocyclohexane$ are another pair of enantiomers, where enantiomers are a type of stereoisomers which are non-superimposable mirror images of each other.
Note: Make sure to draw the structure using IUPAC Nomenclature, if not followed it may lead to errors. Also remember to calculate the total number of stereoisomers at first so that you know how many stereoisomers are possible for a compound. Do not double count the ones which are homomer to each other.
Complete step-by-step answer:The given molecule is $1 - bromo - 2 - chlorocyclohexane$ which can be represented as,

Now we know that for a given molecule the total number of stereoisomers $ = \,{2^n}$, where $n$ is the number of chiral centers.
From the structure we can see there are only two chiral centers present in the molecule, the two chiral centers being ${C_1}$ and ${C_2}$.
So using the formula we get,
Total number of stereoisomers for $1 - bromo - 2 - chlorocyclohexane$ $ = \,\,{2^2}\,\, = \,\,4$.
So there are in total four stereoisomers possible for the given compound, in which in one stereoisomer both the substituents are present above the plane $($denoted by wedge$)$ while in another both the substituents are present below the plane$($denoted by dash$)$, while in other two either one of them is present below the plane and the other above the plane. So the structures of all the possible stereoisomers are:

$\left( {1R,2S} \right) - 1 - bromo - 2 - chlorocyclohexane$

$\left( {1S,2R} \right) - 1 - bromo - 2 - chlorocyclohexane$

$\left( {1R,2R} \right) - 1 - bromo - 2 - chlorocyclohexane$

$\left( {1S,2S} \right) - 1 - bromo - 2 - chlorocyclohexane$
Additional Information:Among the four stereoisomers, $\left( {1R,2S} \right) - 1 - bromo - 2 - chlorocyclohexane$ and $\left( {1S,2R} \right) - 1 - bromo - 2 - chlorocyclohexane$ are a pair of enantiomers while $\left( {1R,2R} \right) - 1 - bromo - 2 - chlorocyclohexane$ and $\left( {1S,2S} \right) - 1 - bromo - 2 - chlorocyclohexane$ are another pair of enantiomers, where enantiomers are a type of stereoisomers which are non-superimposable mirror images of each other.
Note: Make sure to draw the structure using IUPAC Nomenclature, if not followed it may lead to errors. Also remember to calculate the total number of stereoisomers at first so that you know how many stereoisomers are possible for a compound. Do not double count the ones which are homomer to each other.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




