
Draw a neat labelled diagram of ${H_2} - {O_2}$ fuel cell. Write the reactions that occur at the cathode of the cell.
Answer
559.8k+ views
Hint: In a fuel cell, electrical energy gets converted from chemical potential energy (energy stored in molecular bonds). A Proton Exchange Membrane (PEM) cell uses hydrogen gas (\[{H_2}\]) and oxygen gas (\[{O_2}\]) as fuel. The products of the reaction are water, heat, and electricity.
Complete step by step answer:
The diagram of ${H_2} - {O_2}$ fuel cell is shown below:
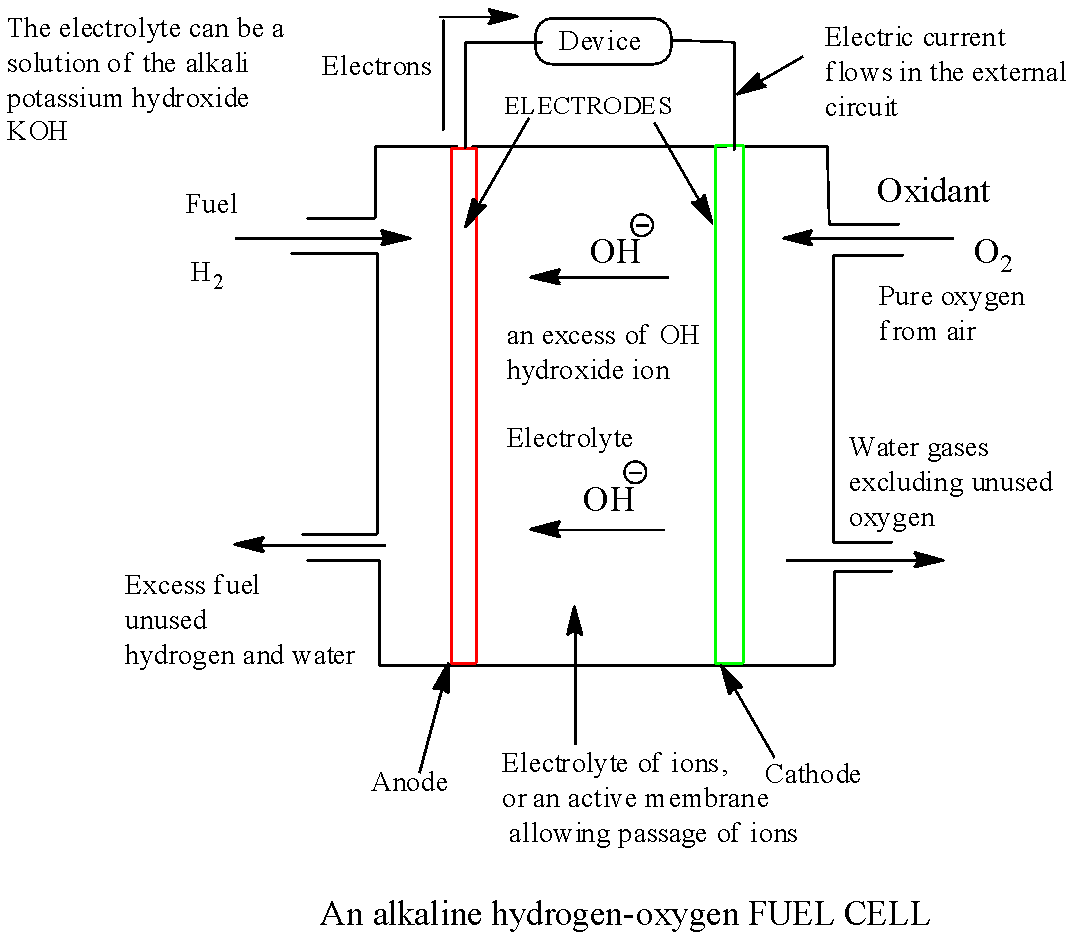
The reaction occurs at cathode of the cell is given below:
The Reducing process at cathode: Oxygen gas water molecules are reduced to Hydroxide ions:
\[{O_2}(g) + 2{H_2}_O(l) + 4{e^ - } \to 4O{H^ - }(aq)\].
Fuel cells are galvanic cells in which the energy of combustion of fuels is directly converted into the electrical energy. This Fuel Cell is also known as ${H_2} - {O_2}$ cell. In this cell the commonly used fuels are methanol, hydrogen etc.
The cell reactions in ${H_2} - {O_2}$ cell takes place at both the electrodes that are at cathodes and anodes. At Cathodes the reduction process takes place while on an Anode the Oxidation process takes place. The reaction at the both the terminals is explained below:
At Anode: The reaction (Oxidation) which takes place at anode is given below:
\[2{H_2}(g) + 4O{H^ - }(aq) \to 4{H_{2}}O(l) + 4{e^ - }2{H_2}(g) + 4O{H^ - }(aq) \to 4{H_2}O(l) + 4{e^ - }\]
In this reaction Hydrogen Gas and hydroxide ions are oxidized to form water. The valency of all the components are taken care of.
At cathode: Reduction process takes place –
\[{O_2}(g) + 2{H_2}O(l) + 4{e^ - } \to 4O{H^ - }(aq)\]
In this reaction Oxygen gas and Water molecules are reduced to Hydroxide ions. The valency of all the elements have been taken care of and fulfilled.
Note: 1) Both Oxidation and Reduction processes take place in the cell reaction.
2) Fuel cells can attain 80% of the energy efficiency.
3) Fuel cells are significantly lighter and more compact.
4) Hydrogen is expensive to produce and not widely available.
5) High cost in manufacturing due to the cost of catalyst (platinum).
6) The ${H_2} - {O_2}$ fuel cell is used as fuel in Space crafts and the end products of the cell reaction are eco friendly.
Complete step by step answer:
The diagram of ${H_2} - {O_2}$ fuel cell is shown below:

The reaction occurs at cathode of the cell is given below:
The Reducing process at cathode: Oxygen gas water molecules are reduced to Hydroxide ions:
\[{O_2}(g) + 2{H_2}_O(l) + 4{e^ - } \to 4O{H^ - }(aq)\].
Fuel cells are galvanic cells in which the energy of combustion of fuels is directly converted into the electrical energy. This Fuel Cell is also known as ${H_2} - {O_2}$ cell. In this cell the commonly used fuels are methanol, hydrogen etc.
The cell reactions in ${H_2} - {O_2}$ cell takes place at both the electrodes that are at cathodes and anodes. At Cathodes the reduction process takes place while on an Anode the Oxidation process takes place. The reaction at the both the terminals is explained below:
At Anode: The reaction (Oxidation) which takes place at anode is given below:
\[2{H_2}(g) + 4O{H^ - }(aq) \to 4{H_{2}}O(l) + 4{e^ - }2{H_2}(g) + 4O{H^ - }(aq) \to 4{H_2}O(l) + 4{e^ - }\]
In this reaction Hydrogen Gas and hydroxide ions are oxidized to form water. The valency of all the components are taken care of.
At cathode: Reduction process takes place –
\[{O_2}(g) + 2{H_2}O(l) + 4{e^ - } \to 4O{H^ - }(aq)\]
In this reaction Oxygen gas and Water molecules are reduced to Hydroxide ions. The valency of all the elements have been taken care of and fulfilled.
Note: 1) Both Oxidation and Reduction processes take place in the cell reaction.
2) Fuel cells can attain 80% of the energy efficiency.
3) Fuel cells are significantly lighter and more compact.
4) Hydrogen is expensive to produce and not widely available.
5) High cost in manufacturing due to the cost of catalyst (platinum).
6) The ${H_2} - {O_2}$ fuel cell is used as fuel in Space crafts and the end products of the cell reaction are eco friendly.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




