
Draw a neat and labelled diagram of $pH$ scale.
Answer
605.4k+ views
Hint : We all are familiar with the term $pH$ and $pH$ scale. $pH$ scale is a scale of acidity and basicity which tells about the acidic and alkaline behaviour of substance .
Complete answer:
> On the $pH$ scale the value ranges from $0$ to $14$. So the more acidic solution will have lower $pH$ and neutral solutions will have $pH$ equal to $7$ and more than $7$ acidic nature decreases and alkaline nature increases .Since $pH$ is defined as the negative of the base $10$ logarithm of the concentration of hydrogen ion ; \[\]$pH$$ = $ $ - {\log _{10}}[{H^ + }]$means $pH$ is a measure of the concentration of ${H^ + }$ ion in the solution.
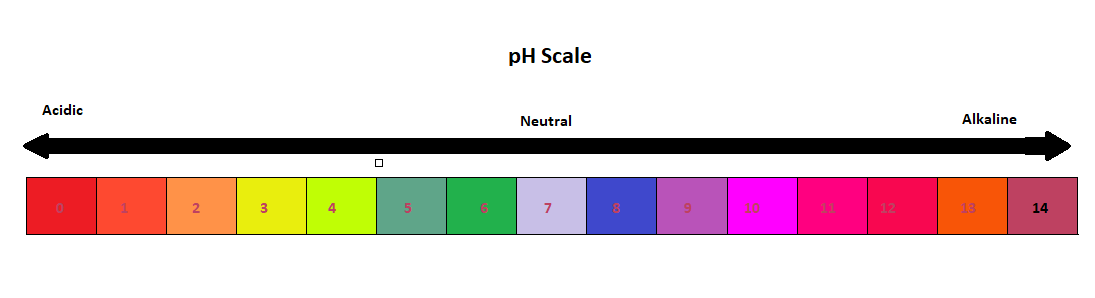 > The $pH$ value can be less than zero which indicates very strong acid and $pH$ value greater than $14$ for a very strong base. A solution where are more ${H^ + }$ ions than $O{H^ - }$ then the solution is acidic and in neutral solution there are equal number of ${H^ + }$ ions and $O{H^ - }$ ions. $pH$ scale has a variety of applications in agriculture, research development, water treatment, environmental monitoring, in quantitative measurement and industrial processing. $pH$ values are affected by temperature so $pH$ applications need to form some temperature compensation to insure standardizes of $pH$ values. We use $pH$ indicators to check the nature of the solution but it does not give precise value of $pH$ because it just changes colour while pH measurement meters used nowadays give an exact value of $pH$.
> The $pH$ value can be less than zero which indicates very strong acid and $pH$ value greater than $14$ for a very strong base. A solution where are more ${H^ + }$ ions than $O{H^ - }$ then the solution is acidic and in neutral solution there are equal number of ${H^ + }$ ions and $O{H^ - }$ ions. $pH$ scale has a variety of applications in agriculture, research development, water treatment, environmental monitoring, in quantitative measurement and industrial processing. $pH$ values are affected by temperature so $pH$ applications need to form some temperature compensation to insure standardizes of $pH$ values. We use $pH$ indicators to check the nature of the solution but it does not give precise value of $pH$ because it just changes colour while pH measurement meters used nowadays give an exact value of $pH$.
Note : Hence $pH$ scale gives information about the acidity and alkalinity of any substance and it ranges from $0$ to $14$ . $pH$ scale is also called the $pH$-acid-base scale. Solutions which are neither acidic nor basic are $7$. So $pH$ is a measure of the hydrogen ion activity in aqueous solution.
Complete answer:
> On the $pH$ scale the value ranges from $0$ to $14$. So the more acidic solution will have lower $pH$ and neutral solutions will have $pH$ equal to $7$ and more than $7$ acidic nature decreases and alkaline nature increases .Since $pH$ is defined as the negative of the base $10$ logarithm of the concentration of hydrogen ion ; \[\]$pH$$ = $ $ - {\log _{10}}[{H^ + }]$means $pH$ is a measure of the concentration of ${H^ + }$ ion in the solution.

Note : Hence $pH$ scale gives information about the acidity and alkalinity of any substance and it ranges from $0$ to $14$ . $pH$ scale is also called the $pH$-acid-base scale. Solutions which are neither acidic nor basic are $7$. So $pH$ is a measure of the hydrogen ion activity in aqueous solution.
Recently Updated Pages
Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Computer Science: Engaging Questions & Answers for Success

Class 10 Question and Answer - Your Ultimate Solutions Guide

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Trending doubts
A boat goes 24 km upstream and 28 km downstream in class 10 maths CBSE

State and explain Ohms law class 10 physics CBSE

Write a letter to the editor of a newspaper explaining class 10 english CBSE

Distinguish between soap and detergent class 10 chemistry CBSE

a Why did Mendel choose pea plants for his experiments class 10 biology CBSE

What is a "free hit" awarded for in limited-overs cricket?




