
Why does the Lewis structure of $Xe{{F}_{4}}$ not follow the octet rule?
Answer
494.1k+ views
Hint: The valence electrons of an atom are represented by Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams). Lewis structures (sometimes called Lewis dot structures or electron dot structures) are diagrams that show how the valence electrons of atoms in a molecule are represented. The valence electrons of atoms and molecules, whether they exist as lone pairs or inside bonds, may be visualised using these Lewis symbols and Lewis structures.
Complete answer:
The octet rule is a chemical rule of thumb that represents the hypothesis that main-group elements tend to bind in such a way that each atom has eight electrons in its valence shell, resulting in an electronic configuration similar to that of a noble gas. The law applies to carbon, nitrogen, oxygen, and halogens in particular, but also to metals like sodium and magnesium. A Lewis electron dot diagram can be used to count the valence electrons.
The chemical compound Xenon Tetrafluoride has the formula $Xe{{F}_{4}}$. It was the first noble gas binary compound to be found. The chemical interaction of xenon with fluorine, \[{{F}_{2}}\], produces it, according to the chemical equation:
\[Xe\text{ }+\text{ }2~{{F}_{2}}~\to ~Xe{{F}_{4}}\]
This reaction is exothermic, producing 251 kJ/mol of energy.
The octet rule dictates that atoms aim to fill their outer valence shell with 8 electrons, however this molecule violates the norm by accumulating 12 total electrons in its quest for a formal charge of 0.
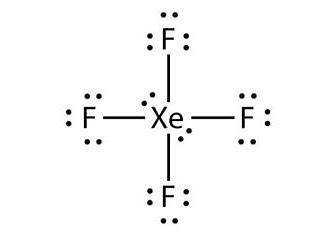
This molecule's Lewis Structure is.
According to the octet rule, atoms prefer to be surrounded by 8 electrons in order to fill their valence shells. This rule is not a law, and there are few exceptions for transition metals, but it is reasonably dependable for noble gases and group period 1-3 elements.
Using this criterion, we can see that Xe has four bonding and two nonbonding pairs of electrons, which it shares and does not share with the fluorine atoms.
It has a formal charge of 0 but is surrounded by 12 electrons. As a result, it deviates from the octet rule.
Note:
Xenon tetrafluoride is a crystalline compound that is colourless. In 1963, NMR spectroscopy and X-ray crystallography were used to establish its structure. Neutron diffraction investigations have shown that the structure is square planar. The xenon centre possesses two lone pairs of electrons, according to VSEPR theory, in addition to four fluoride ligands. These lone pairings are transgender.
Complete answer:
The octet rule is a chemical rule of thumb that represents the hypothesis that main-group elements tend to bind in such a way that each atom has eight electrons in its valence shell, resulting in an electronic configuration similar to that of a noble gas. The law applies to carbon, nitrogen, oxygen, and halogens in particular, but also to metals like sodium and magnesium. A Lewis electron dot diagram can be used to count the valence electrons.
The chemical compound Xenon Tetrafluoride has the formula $Xe{{F}_{4}}$. It was the first noble gas binary compound to be found. The chemical interaction of xenon with fluorine, \[{{F}_{2}}\], produces it, according to the chemical equation:
\[Xe\text{ }+\text{ }2~{{F}_{2}}~\to ~Xe{{F}_{4}}\]
This reaction is exothermic, producing 251 kJ/mol of energy.
The octet rule dictates that atoms aim to fill their outer valence shell with 8 electrons, however this molecule violates the norm by accumulating 12 total electrons in its quest for a formal charge of 0.
This molecule's Lewis Structure is.
According to the octet rule, atoms prefer to be surrounded by 8 electrons in order to fill their valence shells. This rule is not a law, and there are few exceptions for transition metals, but it is reasonably dependable for noble gases and group period 1-3 elements.
Using this criterion, we can see that Xe has four bonding and two nonbonding pairs of electrons, which it shares and does not share with the fluorine atoms.
It has a formal charge of 0 but is surrounded by 12 electrons. As a result, it deviates from the octet rule.

Note:
Xenon tetrafluoride is a crystalline compound that is colourless. In 1963, NMR spectroscopy and X-ray crystallography were used to establish its structure. Neutron diffraction investigations have shown that the structure is square planar. The xenon centre possesses two lone pairs of electrons, according to VSEPR theory, in addition to four fluoride ligands. These lone pairings are transgender.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




