
How does $S{{O}_{4}}$ have a charge of -2?
Answer
564.9k+ views
Hint:We can solve this question after defining the term oxidation number and then we will discuss the relation between oxidation number and charge. Then we know that the total charge on a neutral compound is always equal to zero.
Complete answer:
Oxidation state of sulphur is = +6
Oxidation state of oxygen is = -2
The number of valence electrons in sulphur = 6
And the number of valence electrons in oxygen = 6
Total number of valence electrons in the compound
\[\begin{align}
& =(6\times 1)+(6\times 4) \\
& =30 \\
\end{align}\]
And we know that for 4 full outer shell electronic configurations we would need 32 valence electrons but here we only have 4 electrons. Hence the charge for the given ion is -2. The structure of $S{{O}_{4}}$ having 2- charge is mentioned below:
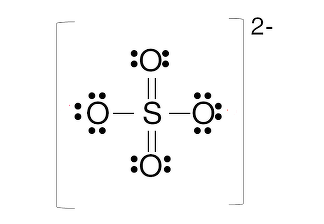
Hence, the charge on $S{{O}_{4}}$ is -2.
Additional information:
In the year 1904, Richard Abegg proposed the concept of coordination number and said that the atoms behave as donors or acceptors of electrons. The octet rule uses this idea and states that every atom binds with another atom to have eight electrons in its outermost shell.
The octet rule is able to explain the formation of a number of compounds and also able to explain the stability of the compound.
Note:
The octet rule is based on the concept that electronic configuration of the noble gases are most stable and they have eight electrons in their outermost orbital so every element should attain the similar electronic configuration to become stable.
Complete answer:
Oxidation state of sulphur is = +6
Oxidation state of oxygen is = -2
The number of valence electrons in sulphur = 6
And the number of valence electrons in oxygen = 6
Total number of valence electrons in the compound
\[\begin{align}
& =(6\times 1)+(6\times 4) \\
& =30 \\
\end{align}\]
And we know that for 4 full outer shell electronic configurations we would need 32 valence electrons but here we only have 4 electrons. Hence the charge for the given ion is -2. The structure of $S{{O}_{4}}$ having 2- charge is mentioned below:

Hence, the charge on $S{{O}_{4}}$ is -2.
Additional information:
In the year 1904, Richard Abegg proposed the concept of coordination number and said that the atoms behave as donors or acceptors of electrons. The octet rule uses this idea and states that every atom binds with another atom to have eight electrons in its outermost shell.
The octet rule is able to explain the formation of a number of compounds and also able to explain the stability of the compound.
Note:
The octet rule is based on the concept that electronic configuration of the noble gases are most stable and they have eight electrons in their outermost orbital so every element should attain the similar electronic configuration to become stable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




