
How does isopropyl alcohol differ from water in polarity?
Answer
548.4k+ views
Hint: Isopropyl alcohol is the simplest example of secondary alcohols. Polarity is defined as the dipole moment. It occurs with a negatively charged end and a positively charged end. Polar molecules must have polar bonds in the structure which make them polar.
Complete step by step answer:
In order to determine the polarity of isopropyl alcohol we have to consider its structure. In the structure of isopropyl alcohol, one of the hydrogen atoms is replaced by a hydrocarbon group. Generally, hydrocarbons are nonpolar in nature due to the low polarity of the hydrocarbon group causing low polarity in the isopropyl alcohol and make it less polar.
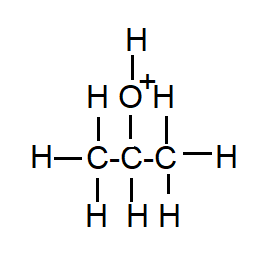
But in the case of water, the structure of water has two hydrogen atoms attached to one oxygen atom. In the molecule of water the bonds are attached in such a way that the dipoles of oxygen-hydrogen bonds add constructively. Due to the difference in electronegativity of oxygen and hydrogen this molecule is much more polar.
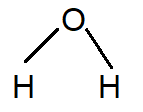
That’s why isopropyl alcohol is less polar than water.
Additional information:
In a molecule there is one or more chemical bonds present between molecular orbitals of different atoms. Polarity of molecules depends on the electronegativity difference between the bonds as explained above. Polarity also occurs due to an asymmetric arrangement of nonpolar covalent bonds and non-bonding pairs of electrons known as a full molecular orbital.
Note: In more simple words, polarity is also called separation of charge because electrons consist of negative charge and the unequal sharing of electrons in a bond causes the formation of electric dipole that is a separation of positive and negative electric charge.
Complete step by step answer:
In order to determine the polarity of isopropyl alcohol we have to consider its structure. In the structure of isopropyl alcohol, one of the hydrogen atoms is replaced by a hydrocarbon group. Generally, hydrocarbons are nonpolar in nature due to the low polarity of the hydrocarbon group causing low polarity in the isopropyl alcohol and make it less polar.

But in the case of water, the structure of water has two hydrogen atoms attached to one oxygen atom. In the molecule of water the bonds are attached in such a way that the dipoles of oxygen-hydrogen bonds add constructively. Due to the difference in electronegativity of oxygen and hydrogen this molecule is much more polar.

That’s why isopropyl alcohol is less polar than water.
Additional information:
In a molecule there is one or more chemical bonds present between molecular orbitals of different atoms. Polarity of molecules depends on the electronegativity difference between the bonds as explained above. Polarity also occurs due to an asymmetric arrangement of nonpolar covalent bonds and non-bonding pairs of electrons known as a full molecular orbital.
Note: In more simple words, polarity is also called separation of charge because electrons consist of negative charge and the unequal sharing of electrons in a bond causes the formation of electric dipole that is a separation of positive and negative electric charge.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




