
: How does benzene differ from other cyclic hydrocarbons?
Answer
542.7k+ views
Hint: Aromatic compounds and the compounds that possess an alternate double and single bond system, where delocalization of electrons can occur.
Complete step-by-step answer:The compound with the formula\[{{C}_{6}}{{H}_{6}}\], contains an alignment with alternate single and double bond inside as ring structure. This compound is known as benzene. Benzene has an aromatic nature, this is proven by the fact that, when we burn it or does a flame test, it burns with a smoky flame; this is the property of aromatic compounds. While, aliphatic compounds burns with flame that is does not produce smoke. Also due to its high carbon content, it has sooty deposits while burning.
Benzene has a cyclic and a planar structure with a six-member carbon ring. The structure of benzene allows delocalization of electrons. The structure is as follows:
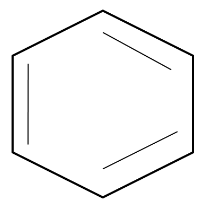
As benzene allows complete delocalization of electrons (pi-electrons), it has conjugation that allows it to participate in electrophilic substitution reactions. This property is possessed by benzene and not by other cyclic hydrocarbons due to absence of aromaticity.
The electrophilic substitution reactions of benzene occur as when an electrophile attacks the benzene, hydrogen gets removed, in the intermediate step, the benzene cation formed by removal of hydrogen is highly reactive, that allows electrophilic substitution on the place of removal of hydrogen. Nitration, sulphonation, Friedal-crafts reactions, are some of the examples of electrophilic substitution reactions of benzene.
Hence, benzene differs in aromaticity and electrophilic substitution reactions from other cyclic hydrocarbons.
Note: Benzene has all the carbons as $s{{p}^{2}}$ hybridized. All the bond lengths are equal in benzene due to resonance, this makes it highly stable and easily available for electrophilic substitution reaction.
Complete step-by-step answer:The compound with the formula\[{{C}_{6}}{{H}_{6}}\], contains an alignment with alternate single and double bond inside as ring structure. This compound is known as benzene. Benzene has an aromatic nature, this is proven by the fact that, when we burn it or does a flame test, it burns with a smoky flame; this is the property of aromatic compounds. While, aliphatic compounds burns with flame that is does not produce smoke. Also due to its high carbon content, it has sooty deposits while burning.
Benzene has a cyclic and a planar structure with a six-member carbon ring. The structure of benzene allows delocalization of electrons. The structure is as follows:

As benzene allows complete delocalization of electrons (pi-electrons), it has conjugation that allows it to participate in electrophilic substitution reactions. This property is possessed by benzene and not by other cyclic hydrocarbons due to absence of aromaticity.
The electrophilic substitution reactions of benzene occur as when an electrophile attacks the benzene, hydrogen gets removed, in the intermediate step, the benzene cation formed by removal of hydrogen is highly reactive, that allows electrophilic substitution on the place of removal of hydrogen. Nitration, sulphonation, Friedal-crafts reactions, are some of the examples of electrophilic substitution reactions of benzene.
Hence, benzene differs in aromaticity and electrophilic substitution reactions from other cyclic hydrocarbons.
Note: Benzene has all the carbons as $s{{p}^{2}}$ hybridized. All the bond lengths are equal in benzene due to resonance, this makes it highly stable and easily available for electrophilic substitution reaction.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




