
Why does acetylation of \[-N{H_2}\] group of aniline reduce its activity?
Answer
601.5k+ views
Hint:
The electron-donating and electron-withdrawing power of the attached functional group is responsible for the activation and deactivation of the phenyl ring. The electron-donating functional group enriches the electron density of the phenyl ring and the electron-withdrawing functional group pulls the electron density of the phenyl group.
Complete answer:
Let us start with aniline and the reason for its increased activity of the phenyl group. In aniline, an amine \[-N{H_2}\] functional group is attached to a phenyl ring. The presence of lone pairs on N undergoes delocalization in the phenyl ring by a phenomenon called resonance. It can be shown as:
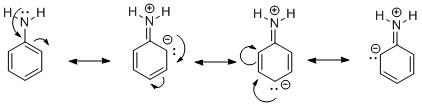
Due to the movement of electron pairs in the phenyl ring, the ring becomes very reactive towards electron-deficient electrophiles. Thus the amine group increases the activity of the phenyl group.
But when the amine group undergoes acetylation, the activity of the phenyl group reduces. We know that the acetyl group contains a carbonyl group which is electron-deficient and attracts the lone pair of electrons present on N towards itself. As a result, the lone pair of N can no longer take part in resonance with the phenyl ring. It rather moves towards the more electron-deficient carbonyl group of acetyl. This is shown as:
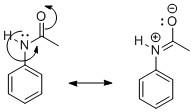
Due to the delocalization of electron pairs in the carbonyl functional group, the nucleophilicity of the phenyl group decreases. As a result, its reactivity decreases. That is why acetylation of the \[-N{H_2}\] group of aniline reduces its activity.
Note:
If the alkylation acetanilide is performed, then an O-alkylated side product is generated which clearly indicates the presence of \[{O^ - }\] nucleophile in acetanilide. Other electron-donating groups are \[OH\], \[OMe\], \[Me\] etc which increase the activity of phenyl ring while \[N{O_2}\], \[COOH\], \[CN\] etc decreases the activity of phenyl ring.
The electron-donating and electron-withdrawing power of the attached functional group is responsible for the activation and deactivation of the phenyl ring. The electron-donating functional group enriches the electron density of the phenyl ring and the electron-withdrawing functional group pulls the electron density of the phenyl group.
Complete answer:
Let us start with aniline and the reason for its increased activity of the phenyl group. In aniline, an amine \[-N{H_2}\] functional group is attached to a phenyl ring. The presence of lone pairs on N undergoes delocalization in the phenyl ring by a phenomenon called resonance. It can be shown as:

Due to the movement of electron pairs in the phenyl ring, the ring becomes very reactive towards electron-deficient electrophiles. Thus the amine group increases the activity of the phenyl group.
But when the amine group undergoes acetylation, the activity of the phenyl group reduces. We know that the acetyl group contains a carbonyl group which is electron-deficient and attracts the lone pair of electrons present on N towards itself. As a result, the lone pair of N can no longer take part in resonance with the phenyl ring. It rather moves towards the more electron-deficient carbonyl group of acetyl. This is shown as:

Due to the delocalization of electron pairs in the carbonyl functional group, the nucleophilicity of the phenyl group decreases. As a result, its reactivity decreases. That is why acetylation of the \[-N{H_2}\] group of aniline reduces its activity.
Note:
If the alkylation acetanilide is performed, then an O-alkylated side product is generated which clearly indicates the presence of \[{O^ - }\] nucleophile in acetanilide. Other electron-donating groups are \[OH\], \[OMe\], \[Me\] etc which increase the activity of phenyl ring while \[N{O_2}\], \[COOH\], \[CN\] etc decreases the activity of phenyl ring.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




