
Distinguish between following:
Benzaldehyde and benzoic acid
Answer
581.7k+ views
Hint: Benzaldehyde and benzoic acid both are aromatic compounds, the difference is the group attached to the benzene ring. The functional group for aldehyde is –CHO and the functional group of carboxylic acid is –COOH.
Complete answer:
Benzaldehyde: Benzaldehyde is the simplest aromatic aldehyde which consists of a benzene ring joined with a single formyl group. The other name for benzoic acid is benzenedicarboxaldehyde. By the suffix itself it can be recognized that it is an aldehydic compound.
The structure of benzaldehyde is shown below.
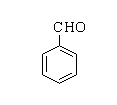
Benzoic acid: Benzoic acid is the simplest aromatic carboxylic acid which consists of a benzene ring joined with a single carboxylic group. The other name for benzoic acid is carboxybenzene. By the suffix –oic itself it can be recognized that it is a carboxylic compound.
The structure of benzoic acid is shown below.
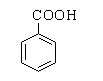
The benzaldehyde can be distinguished from benzoic acid by a sodium bicarbonate test. Benzoic acid is an acid, therefore on reacting with a base sodium bicarbonate it forms a sodium salt of benzoic acid by releasing carbon dioxide gas.
The reaction is shown below.
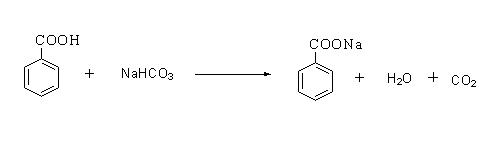
On the other hand, benzaldehyde does not give any reaction with sodium bicarbonate.
Note:
The other test used to distinguish benzaldehyde and benzoic acid is reacting with Tollen’s reagent also known as silver mirror test. Benzaldehyde reacts with ammoniacal solution of silver nitrate to generate silver mirror.
Complete answer:
Benzaldehyde: Benzaldehyde is the simplest aromatic aldehyde which consists of a benzene ring joined with a single formyl group. The other name for benzoic acid is benzenedicarboxaldehyde. By the suffix itself it can be recognized that it is an aldehydic compound.
The structure of benzaldehyde is shown below.

Benzoic acid: Benzoic acid is the simplest aromatic carboxylic acid which consists of a benzene ring joined with a single carboxylic group. The other name for benzoic acid is carboxybenzene. By the suffix –oic itself it can be recognized that it is a carboxylic compound.
The structure of benzoic acid is shown below.

The benzaldehyde can be distinguished from benzoic acid by a sodium bicarbonate test. Benzoic acid is an acid, therefore on reacting with a base sodium bicarbonate it forms a sodium salt of benzoic acid by releasing carbon dioxide gas.
The reaction is shown below.

On the other hand, benzaldehyde does not give any reaction with sodium bicarbonate.
Note:
The other test used to distinguish benzaldehyde and benzoic acid is reacting with Tollen’s reagent also known as silver mirror test. Benzaldehyde reacts with ammoniacal solution of silver nitrate to generate silver mirror.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




