
Discuss the nature of bonding in ${[Fe{(CN)_6}]^{ - 4}}$ on the basis of valence bond theory. What is the structure and magnetic characteristics? (At number Fe = 26).
Answer
573.6k+ views
Hint:From the given complex it can be seen that there is one Fe ion and six cyanide ions. The oxidation number of cyanide ions is +1 and the total charge of the complex is -4, so the oxidation state of the Fe ion can be calculated. Cyanide ion is a strong field ligand.
Complete answer:
The atomic number of iron (Fe) is 26. The outer electronic configuration of Fe is $3{d^6}4{s^2}$. The iron present in the complex ion is in +2 oxidation state, thus the iron loses its two electrons to form $F{e^{2 + }}$ ion. The outer electronic configuration of $F{e^{2 + }}$ is $3{d^6}$.
The ligand present in the complex is cyanide ion which is a strong field ligand which results in pairing.
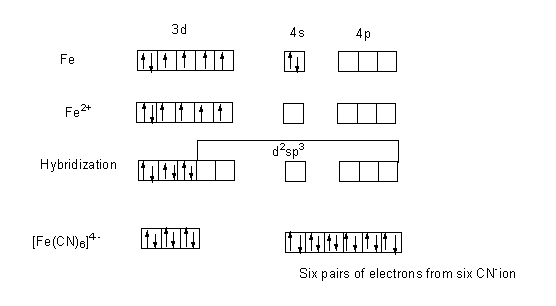
The two electrons of 3d orbital jump and pair up with the two more unpaired electrons in 3d orbital thus leaving two empty d-orbital, one s-orbital and three p-orbital. Thus the resulting hybridization will be ${d^2}s{p^3}$. The empty orbital is filled with six pairs of electrons from $C{N^ - }$ ion.
As, no unpaired electron is present in any orbital, therefore the compound is diamagnetic.
The complex ion formed ${[Fe{(CN)_6}]^{ - 4}}$, has octahedral geometry.
Note:
During the bonding of electrons, the pairing is done according to Hund’s rule which states that first every orbital will be filled with single electrons only then pairing of electrons is done.
Complete answer:
The atomic number of iron (Fe) is 26. The outer electronic configuration of Fe is $3{d^6}4{s^2}$. The iron present in the complex ion is in +2 oxidation state, thus the iron loses its two electrons to form $F{e^{2 + }}$ ion. The outer electronic configuration of $F{e^{2 + }}$ is $3{d^6}$.
The ligand present in the complex is cyanide ion which is a strong field ligand which results in pairing.

The two electrons of 3d orbital jump and pair up with the two more unpaired electrons in 3d orbital thus leaving two empty d-orbital, one s-orbital and three p-orbital. Thus the resulting hybridization will be ${d^2}s{p^3}$. The empty orbital is filled with six pairs of electrons from $C{N^ - }$ ion.
As, no unpaired electron is present in any orbital, therefore the compound is diamagnetic.
The complex ion formed ${[Fe{(CN)_6}]^{ - 4}}$, has octahedral geometry.
Note:
During the bonding of electrons, the pairing is done according to Hund’s rule which states that first every orbital will be filled with single electrons only then pairing of electrons is done.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




