
Dipole moment of $C{{O}_{2}}$ molecule is zero whereas $S{{O}_{2}}$ has some dipole moment. Explain the reason.
Answer
570.9k+ views
Hint: Dipole moment is a measure of polarity in a molecule, in other words, the non-polar molecules would have a dipole moment of zero, whereas polar molecules would have other than zero.
There are certain factors which determine whether a molecule would be nonpolar or polar, and one of the major factors is, difference in electronegativity of the constituent atoms in the molecule.
Complete step by step answer:
Dipole moment is a phenomena that occurs when there is a charge separation. Dipole moments can be observed between two different ions with ionic bonds or it could also be observed between atoms which are attached to each other through covalent bonds; dipole moments come into play because of the differences in electronegativity. And with the increase in difference in electronegativity, the dipole moment also increases. The distance which is present between the positive and negative charge separation is also responsible for the size of the dipole moment. The value of dipole moment is a measure of the polarity which is present in the molecule.
Or in other words we can say that when atoms which are constituting a molecule share their electrons in an unequal manner, they establish a dipole moment between them. This happens when one of the constituent atom is more electronegative than the other atom, ultimately resulting in that more electronegative atom pulling more extent of shared pair of electrons between the two atoms, or in another case when one atom has the presence of lone pair of electron in it and the difference of electronegativity vector, points in the same direction. We can consider one of the most commonly known examples of a molecule which shows dipole moment, which is the water molecule, which consists of one atom of oxygen and two atoms of hydrogen. The differences of the two atoms in terms of electronegativity and lone pair of electrons give each hydrogen a partial positive charge and the oxygen atom a partial negative charge.
Now if we consider the two molecules given in the question, the lewis dot structures of both of these molecules should be drawn in order to understand the number of lone pairs of electrons each of the atoms have. So the lewis dot structures of carbon dioxide and sulphur dioxide are given below.
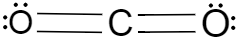
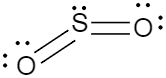
Now if we consider the two structures we can see that in case of carbon dioxide the structure is a linear one because it contains two oxygen and one carbon and their valencies are satisfied by sharing the electrons, and they have a $180{}^\circ $ angle between them. Carbon dioxide is a non-polar molecule because the two oxygen are present on the alternate side of the carbon, cancels out the dipole moments of each other.
While in case of Sulphur dioxide the structure is a bent structure because the Sulphur also has a lone pair of electrons and unlike carbon, these electrons repel the lone pair of electrons which are present on the oxygen. So, because of this repulsion, the structure becomes slightly bent, and the angle becomes $120{}^\circ $. As a result as we can see the sulphur dioxide becomes a polar molecule, as the dipole moments of the two atoms of oxygen which are present in either side of the sulphur are not cancelling out.
Note: Carbon dioxide is a non-polar molecule because it has a linear shape as the dipole moment of the two oxygen present on either side of the carbon cancels out each other, and the carbon doesn’t have any lone pairs in its valence shell.
While in case of sulphur dioxide, its has some value of dipole moment because, the dipole moments of the two oxygens doesn’t cancel out as the sulphur has a lone pair of electron as a result of which the structures acquires a bent shape resulting in non-cancellation of dipole moment, hence a polar molecule.
There are certain factors which determine whether a molecule would be nonpolar or polar, and one of the major factors is, difference in electronegativity of the constituent atoms in the molecule.
Complete step by step answer:
Dipole moment is a phenomena that occurs when there is a charge separation. Dipole moments can be observed between two different ions with ionic bonds or it could also be observed between atoms which are attached to each other through covalent bonds; dipole moments come into play because of the differences in electronegativity. And with the increase in difference in electronegativity, the dipole moment also increases. The distance which is present between the positive and negative charge separation is also responsible for the size of the dipole moment. The value of dipole moment is a measure of the polarity which is present in the molecule.
Or in other words we can say that when atoms which are constituting a molecule share their electrons in an unequal manner, they establish a dipole moment between them. This happens when one of the constituent atom is more electronegative than the other atom, ultimately resulting in that more electronegative atom pulling more extent of shared pair of electrons between the two atoms, or in another case when one atom has the presence of lone pair of electron in it and the difference of electronegativity vector, points in the same direction. We can consider one of the most commonly known examples of a molecule which shows dipole moment, which is the water molecule, which consists of one atom of oxygen and two atoms of hydrogen. The differences of the two atoms in terms of electronegativity and lone pair of electrons give each hydrogen a partial positive charge and the oxygen atom a partial negative charge.
Now if we consider the two molecules given in the question, the lewis dot structures of both of these molecules should be drawn in order to understand the number of lone pairs of electrons each of the atoms have. So the lewis dot structures of carbon dioxide and sulphur dioxide are given below.
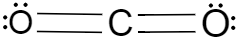
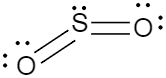
Now if we consider the two structures we can see that in case of carbon dioxide the structure is a linear one because it contains two oxygen and one carbon and their valencies are satisfied by sharing the electrons, and they have a $180{}^\circ $ angle between them. Carbon dioxide is a non-polar molecule because the two oxygen are present on the alternate side of the carbon, cancels out the dipole moments of each other.
While in case of Sulphur dioxide the structure is a bent structure because the Sulphur also has a lone pair of electrons and unlike carbon, these electrons repel the lone pair of electrons which are present on the oxygen. So, because of this repulsion, the structure becomes slightly bent, and the angle becomes $120{}^\circ $. As a result as we can see the sulphur dioxide becomes a polar molecule, as the dipole moments of the two atoms of oxygen which are present in either side of the sulphur are not cancelling out.
Note: Carbon dioxide is a non-polar molecule because it has a linear shape as the dipole moment of the two oxygen present on either side of the carbon cancels out each other, and the carbon doesn’t have any lone pairs in its valence shell.
While in case of sulphur dioxide, its has some value of dipole moment because, the dipole moments of the two oxygens doesn’t cancel out as the sulphur has a lone pair of electron as a result of which the structures acquires a bent shape resulting in non-cancellation of dipole moment, hence a polar molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




