
When dinitrogen trioxide is reacted with a base like NaOH?
Answer
580.8k+ views
Hint: On reaction with sodium hydroxide acidic oxides of nitrogen forms sodium nitrite or sodium nitrate salt along with water. Only one oxide containing an even number of oxygen atoms forms both along with water and most acidic oxide forms only nitrate salt. Only Nitric oxide gives one extra product ${{N}_{2}}O$ with $NaOH$.
Complete answer:
Dinitrogen trioxide is one of the oxides of nitrogen with the formula N2O3. It is a deep blue solid. Its structure is shown below:
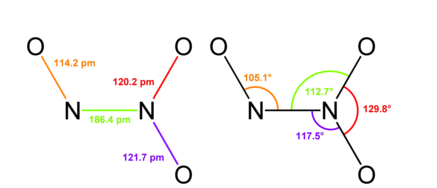
-It forms upon mixing equal parts of \[NO\] and $N{{O}_{2}}$ and cooling the mixture below $-21{}^{0}C$
\[NO+N{{O}_{2}}\rightleftharpoons {{N}_{2}}{{O}_{3}}\]
-Dinitrogen trioxide is only isolable at low temperatures, i.e. in the liquid and solid phases. At higher temperatures the equilibrium favors the constituent gases, with dissociation constant of $193kPa$ at $25{}^{0}C$
-$NaOH$ is called sodium hydroxide and it is a strong base completely soluble in water.
Dinitrogen trioxide reacts with sodium hydroxide to produce sodium nitrite and water
\[{{N}_{2}}{{O}_{3}}+2NaOH\to 2NaN{{O}_{2}}+{{H}_{2}}O\]
-Acidity of oxides of nitrogen increases as the oxidation number of nitrogen increases in them. ${{N}_{2}}O$ and $NO$ are neutral and ${{N}_{2}}O{}_{3}$, $N{{O}_{2}}$ and ${{N}_{2}}{{O}_{5}}$ are acidic in nature with ${{N}_{2}}{{O}_{5}}$ is most acidic among all.
Additional information:
It is the anhydride of the unstable nitrous acid ($HN{{O}_{2}}$) and produces it when mixed into water.
\[{{N}_{2}}{{O}_{3}}+{{H}_{2}}O\to 2HN{{O}_{2}}\]
Note: ${{N}_{2}}{{O}_{3}}$ is a chemical compound formed with a chemical name dinitrogen trioxide. It is also called Nitrogen trioxide, or nitrogen sesquioxide. It is highly a toxic compound and irritating to mucous membranes. It is extremely irritating to skin, mucous membranes, and eyes. Inhaling the vapours is very toxic. It is widely used for certain purpose fuels.
Complete answer:
Dinitrogen trioxide is one of the oxides of nitrogen with the formula N2O3. It is a deep blue solid. Its structure is shown below:

-It forms upon mixing equal parts of \[NO\] and $N{{O}_{2}}$ and cooling the mixture below $-21{}^{0}C$
\[NO+N{{O}_{2}}\rightleftharpoons {{N}_{2}}{{O}_{3}}\]
-Dinitrogen trioxide is only isolable at low temperatures, i.e. in the liquid and solid phases. At higher temperatures the equilibrium favors the constituent gases, with dissociation constant of $193kPa$ at $25{}^{0}C$
-$NaOH$ is called sodium hydroxide and it is a strong base completely soluble in water.
Dinitrogen trioxide reacts with sodium hydroxide to produce sodium nitrite and water
\[{{N}_{2}}{{O}_{3}}+2NaOH\to 2NaN{{O}_{2}}+{{H}_{2}}O\]
-Acidity of oxides of nitrogen increases as the oxidation number of nitrogen increases in them. ${{N}_{2}}O$ and $NO$ are neutral and ${{N}_{2}}O{}_{3}$, $N{{O}_{2}}$ and ${{N}_{2}}{{O}_{5}}$ are acidic in nature with ${{N}_{2}}{{O}_{5}}$ is most acidic among all.
Additional information:
It is the anhydride of the unstable nitrous acid ($HN{{O}_{2}}$) and produces it when mixed into water.
\[{{N}_{2}}{{O}_{3}}+{{H}_{2}}O\to 2HN{{O}_{2}}\]
Note: ${{N}_{2}}{{O}_{3}}$ is a chemical compound formed with a chemical name dinitrogen trioxide. It is also called Nitrogen trioxide, or nitrogen sesquioxide. It is highly a toxic compound and irritating to mucous membranes. It is extremely irritating to skin, mucous membranes, and eyes. Inhaling the vapours is very toxic. It is widely used for certain purpose fuels.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




