
How would you differentiate between monosubstituted and disubstituted alkenes?
Answer
550.8k+ views
Hint: We know that alkene is a hydrocarbon containing one or more Carbon-Carbon double bonds. Some examples of alkene are ethene, butane, etc. Alkenes with 2 to 4 carbon atoms are gases at room temperature.
Complete step by step answer:
Let’s learn the differences between monosubstituted and disubstituted alkene.
Additional information:
Let’s understand some important points of alkene.
1) Most physical and chemical properties of alkenes are similar to those of alkanes.
2) With the increase of molecular mass, the boiling points of the alkenes increases.
3) The increase of branching of alkenes results in greater volatility and lower boiling points.
4) Alkenes can undergo polymerization.
5) Alkenes composed of both sigma and pi bonds.
Let’s discuss some chemical properties of alkenes.
1) Alkenes react with bromine to form vicinal dihalide. Among the halogens, bromine does not undergo reaction with alkenes.
${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}} + {\text{B}}{{\text{r}}_{\text{2}}} \to {{\text{C}}_2}{{\text{H}}_4}{\text{B}}{{\text{r}}_2}$
2) Alkenes undergo reduction reaction with hydrogen in presence of platinum or nickel to form alkanes.
${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}} + {{\text{H}}_{\text{2}}} \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_3}$
Note: It is to be noted that the tetrasubstituted alkene is the alkene in which double bonded carbon atoms are bonded to three other carbon atoms. For example,
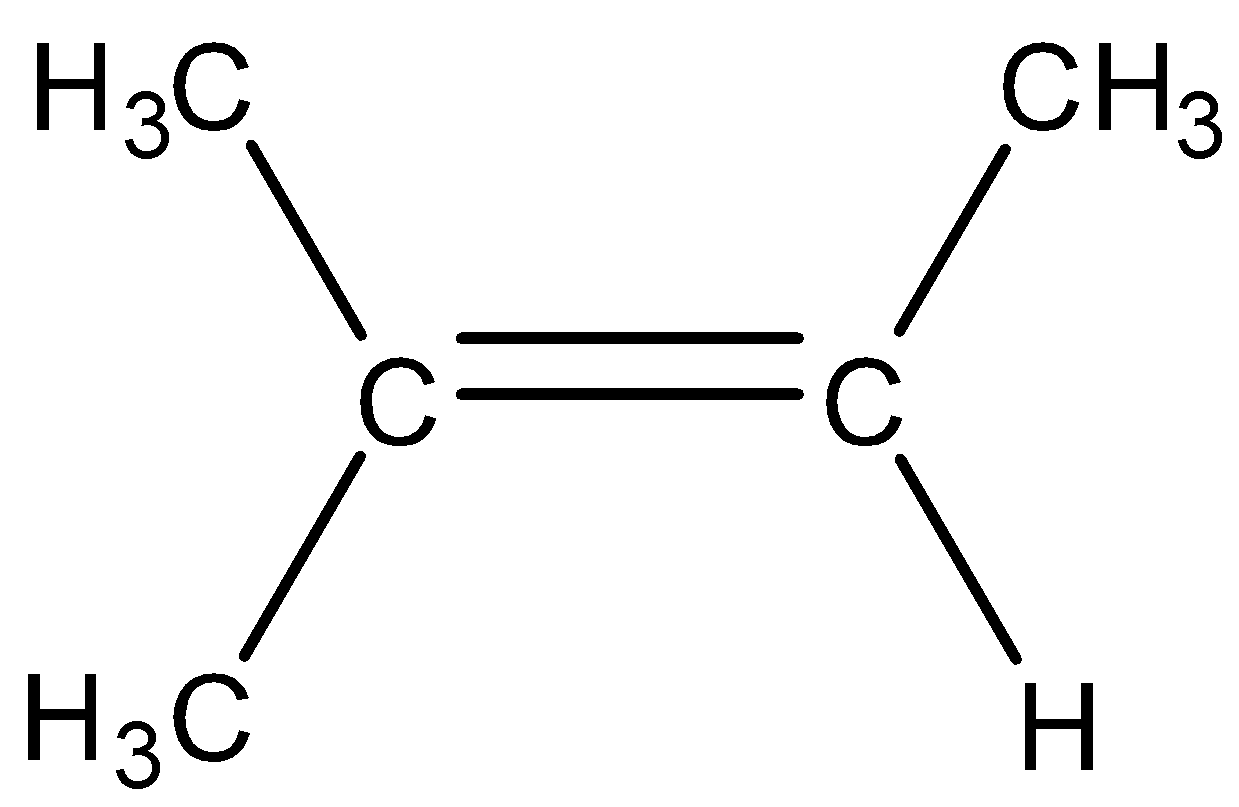
As there are three substitutions, therefore, it is known as tetrasubstituted alkene.
Complete step by step answer:
Let’s learn the differences between monosubstituted and disubstituted alkene.
| Monosubstituted alkene | Disubstituted alkene |
| 1) A monosubstituted alkene is the alkene in which double bonded carbon atoms are bonded to only one carbon atom | 1) It is the alkene in which double bonded carbon atoms are bonded to two other carbon atoms. |
| 2) As only one substitution present, therefore, it is known as monosubstituted alkene. | 2) As there are two substitutions, therefore, it is known as disubstituted alkene. |
3)For example, 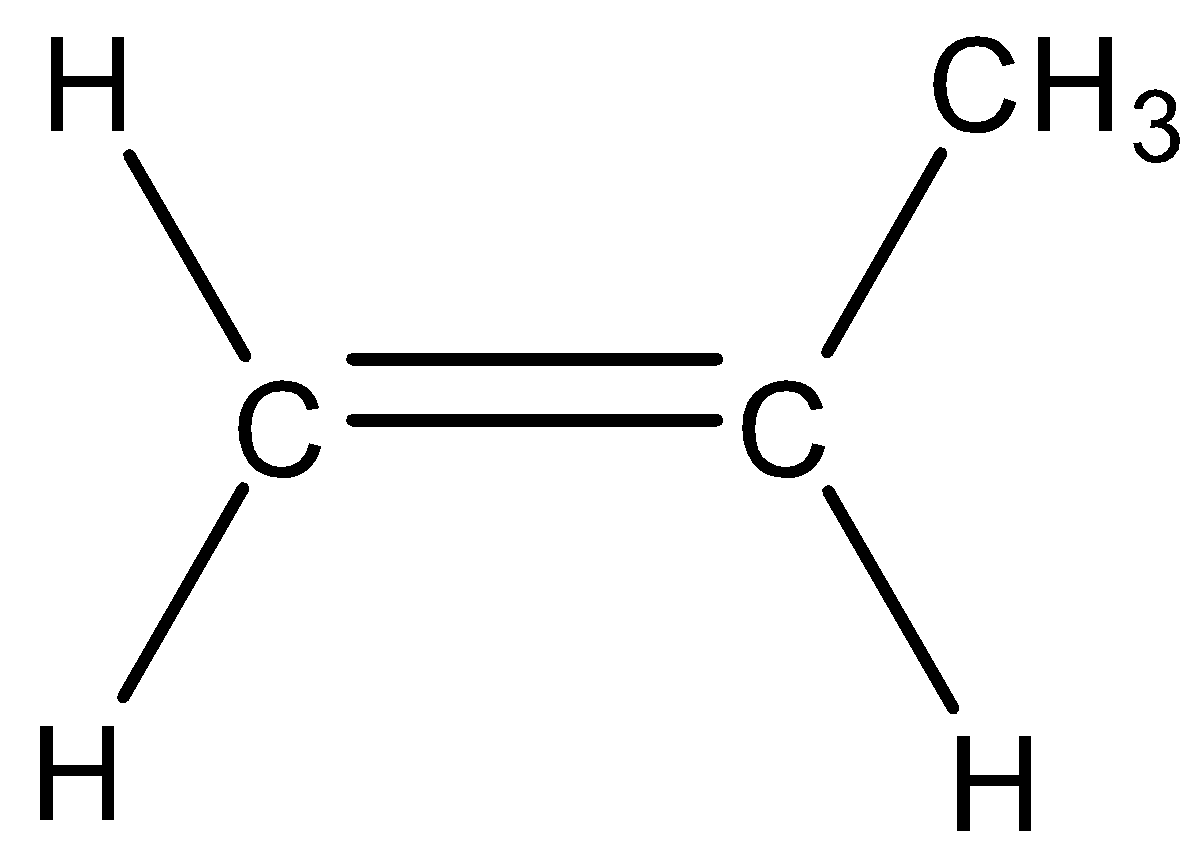
| 3)For example,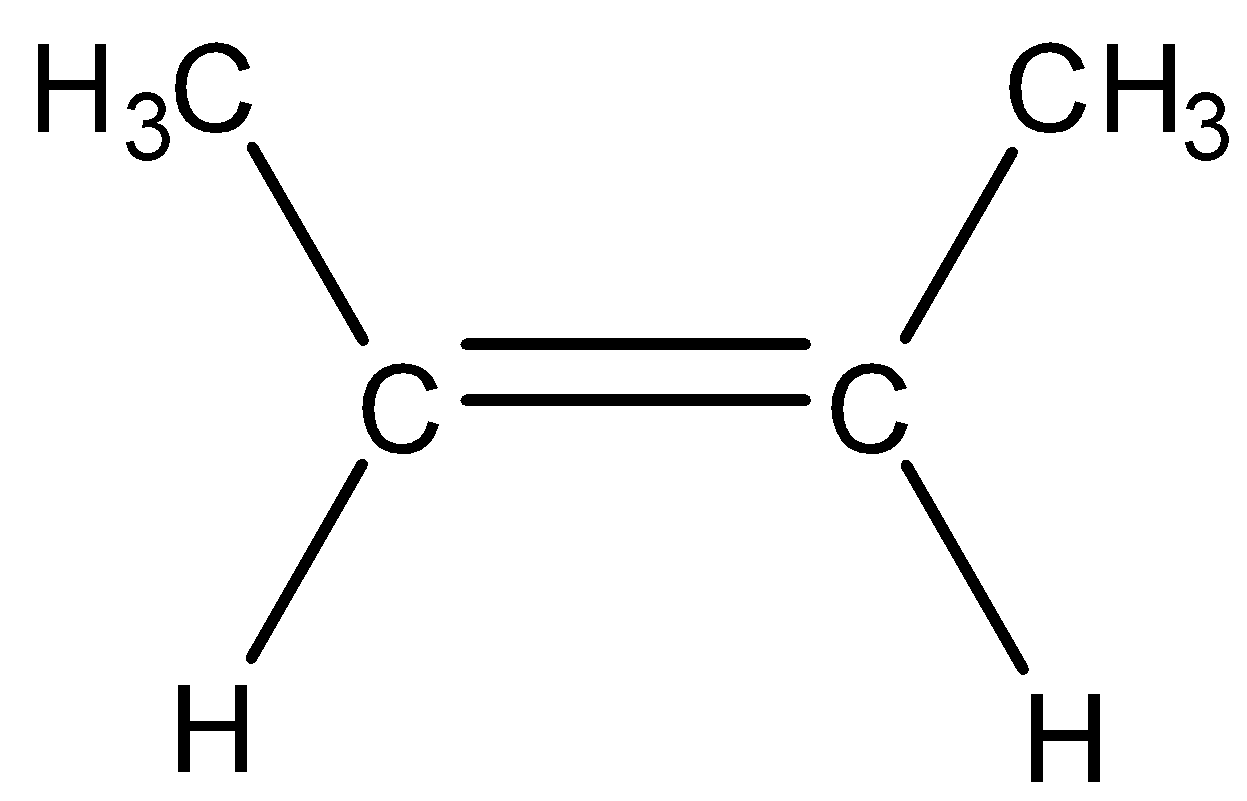
|
| 4) Stability is less | 4)Stability is high |
Additional information:
Let’s understand some important points of alkene.
1) Most physical and chemical properties of alkenes are similar to those of alkanes.
2) With the increase of molecular mass, the boiling points of the alkenes increases.
3) The increase of branching of alkenes results in greater volatility and lower boiling points.
4) Alkenes can undergo polymerization.
5) Alkenes composed of both sigma and pi bonds.
Let’s discuss some chemical properties of alkenes.
1) Alkenes react with bromine to form vicinal dihalide. Among the halogens, bromine does not undergo reaction with alkenes.
${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}} + {\text{B}}{{\text{r}}_{\text{2}}} \to {{\text{C}}_2}{{\text{H}}_4}{\text{B}}{{\text{r}}_2}$
2) Alkenes undergo reduction reaction with hydrogen in presence of platinum or nickel to form alkanes.
${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}} + {{\text{H}}_{\text{2}}} \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_3}$
Note: It is to be noted that the tetrasubstituted alkene is the alkene in which double bonded carbon atoms are bonded to three other carbon atoms. For example,

As there are three substitutions, therefore, it is known as tetrasubstituted alkene.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




