
What is the difference between Hofmann and Curtius rearrangement?
Answer
505.8k+ views
Hint: We need to know that the rearrangement reaction is an organic reaction and here the carbon skeletal present in the molecule is completely rearranged. Both Hofmann and Curtius rearrangement is used for the conversion of chemical compounds. In the case of Hoffmann bromamide reaction, the amide is reacting with bromine in the presence of an ethanolic solution of sodium hydroxide. And in the case of Curtius rearrangement, the amide is decomposed thermally and form corresponding compound by losing of nitrogen atoms.
Complete answer:
We remember that the Hoffmann rearrangement reaction is a chemical reaction which is used for the conversion of primary amide to primary amine having less number of carbon atoms. Let’s see the reaction-
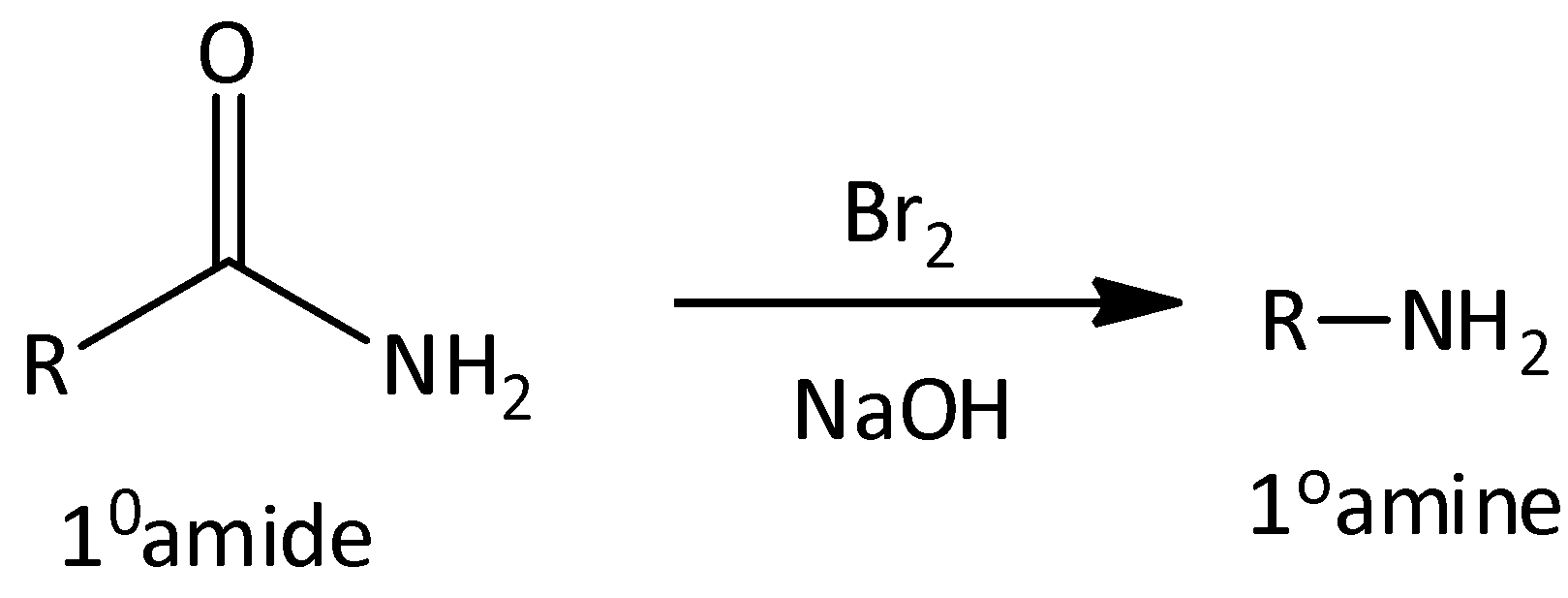
The Curtius rearrangement is a chemical reaction which is used for the conversion of acyl cyanide to isocyanate. Let’s see the reaction-
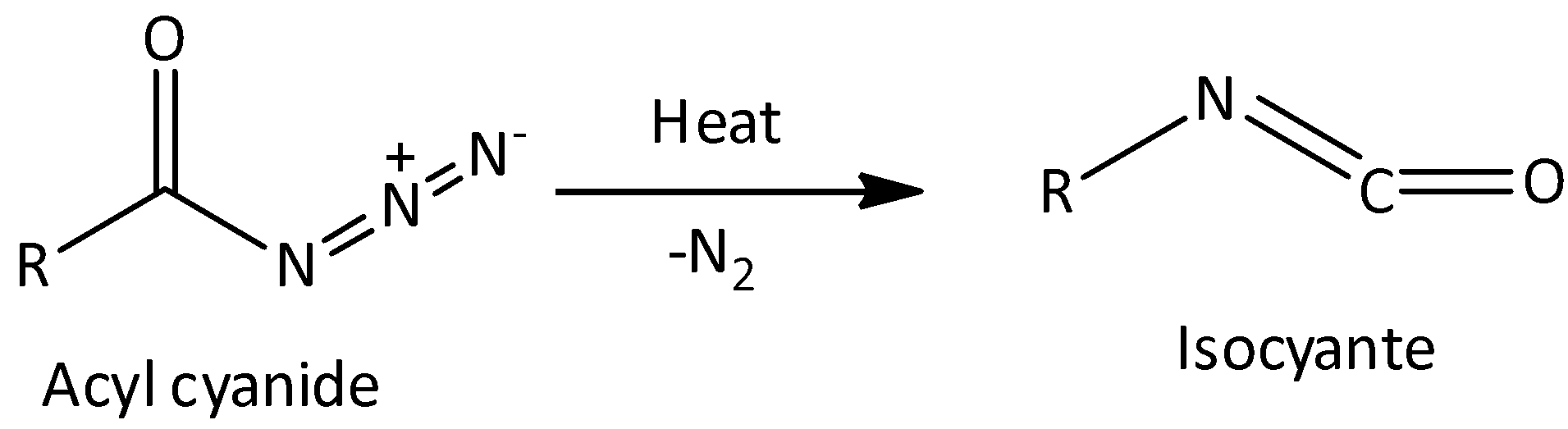
In the Hofmann rearrangement reaction, the reactant is primary amide and product formed is primary amine. And in Curtius rearrangement, the reactant is acyl azide and product is isocyanate.
The released compound in Hofmann rearrangement reaction carbon dioxide. But in the case of Curtius rearrangement, the released compound is nitrogen. These are the difference between Hoffmann rearrangement reaction and Curtius rearrangement.
Note:
We must have to know that the Hoffmann rearrangement is also known as the Hoffmann bromamide degradation reaction. Because this rearrangement assumes the degradation of amide. The number of carbon present in the primary amine is less than the original amide. In Curtius rearrangement, there is a formation of isocyanate from acyl cyanide. And this stable isocyanate is converted into a variety of amines and derivatives of amines.
Complete answer:
We remember that the Hoffmann rearrangement reaction is a chemical reaction which is used for the conversion of primary amide to primary amine having less number of carbon atoms. Let’s see the reaction-
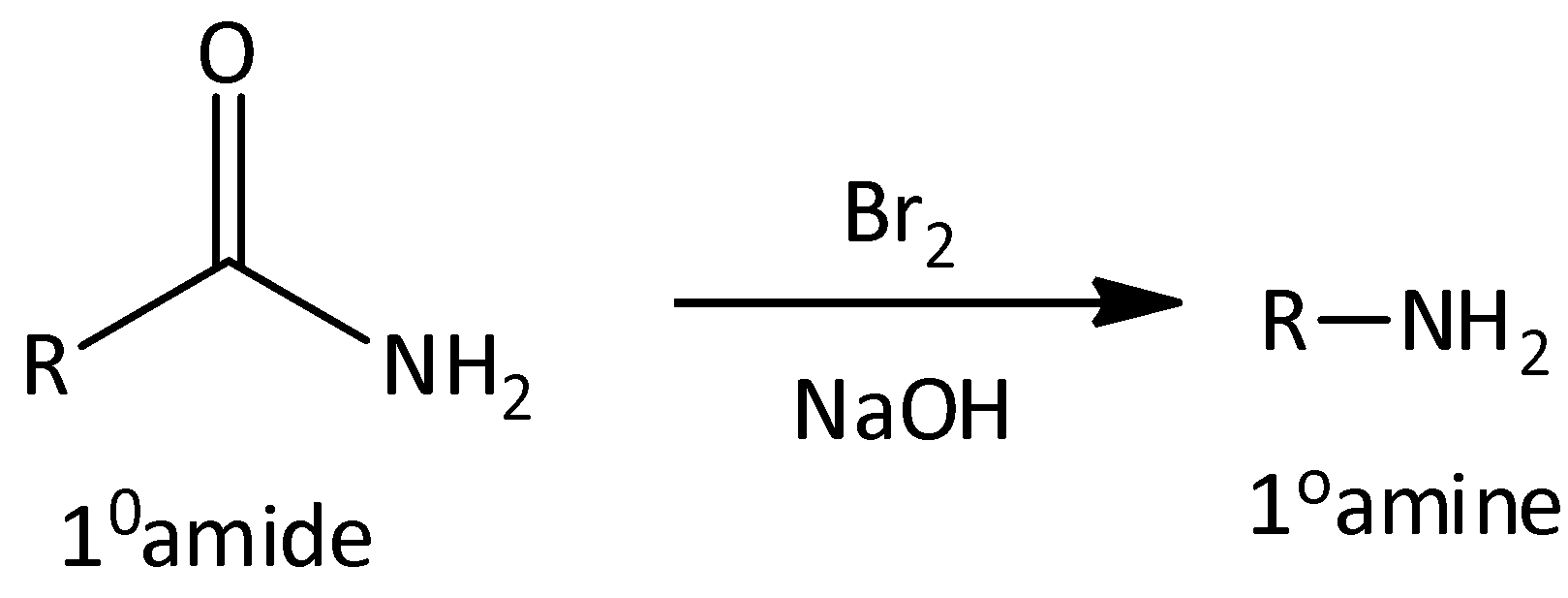
The Curtius rearrangement is a chemical reaction which is used for the conversion of acyl cyanide to isocyanate. Let’s see the reaction-

In the Hofmann rearrangement reaction, the reactant is primary amide and product formed is primary amine. And in Curtius rearrangement, the reactant is acyl azide and product is isocyanate.
The released compound in Hofmann rearrangement reaction carbon dioxide. But in the case of Curtius rearrangement, the released compound is nitrogen. These are the difference between Hoffmann rearrangement reaction and Curtius rearrangement.
Note:
We must have to know that the Hoffmann rearrangement is also known as the Hoffmann bromamide degradation reaction. Because this rearrangement assumes the degradation of amide. The number of carbon present in the primary amine is less than the original amide. In Curtius rearrangement, there is a formation of isocyanate from acyl cyanide. And this stable isocyanate is converted into a variety of amines and derivatives of amines.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




