
How many diastereomers exist for the compound below?
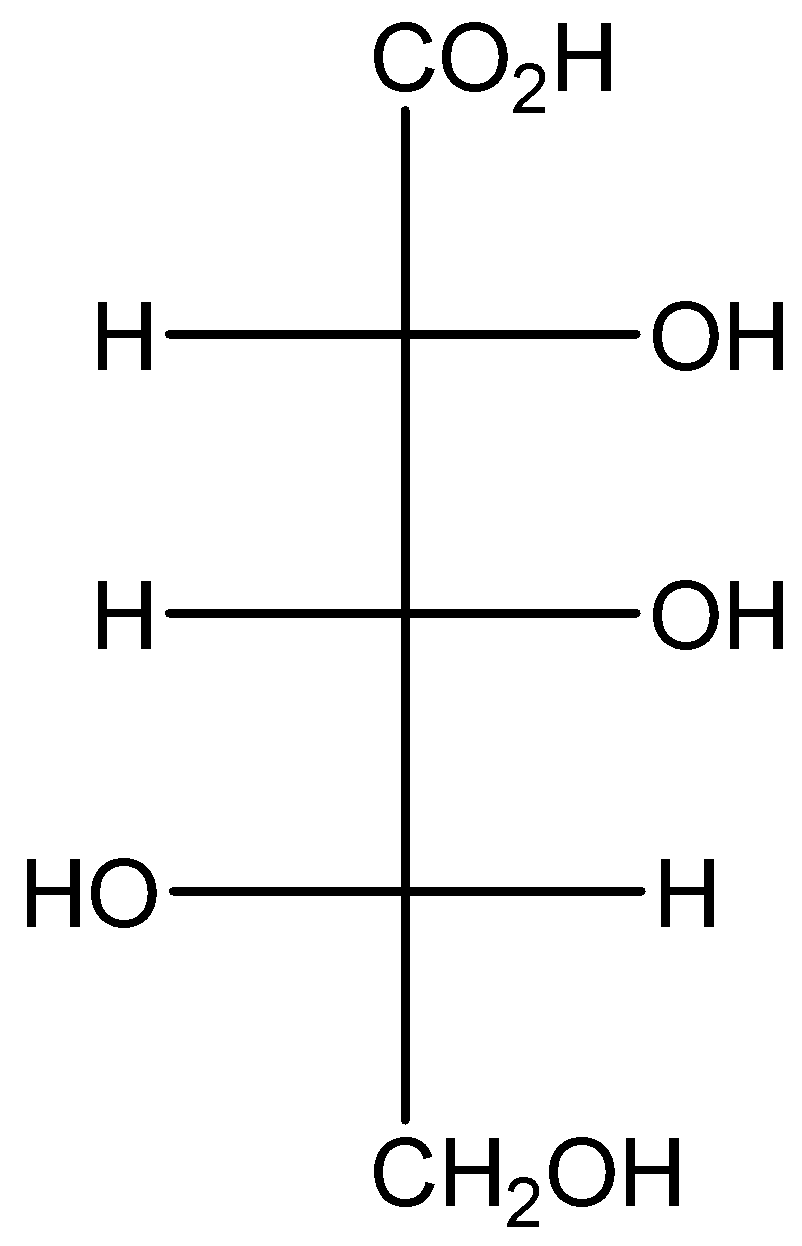
A) \[2\]
B) \[4\]
C) \[6\]
D) \[7\]

Answer
513k+ views
Hint: We have to know that the isomer of the organic molecule is divided by two types. There are constitutional isomers and stereoisomers. Further constitutional isomers are classified as chain isomer, position isomer, functional isomer, Metamers, Tautomer and ring chain isomer. The stereoisomers are classified as geometrical isomer and optical isomer. The isomer means the same molecular formula but different in structural or positional or functional or chain of the carbon atom in the organic molecule.
Formula used:
The number of optical isomers is =\[{{\text{2}}^{\text{n}}}\].
The number of diastereomers is =\[{{\text{2}}^{\text{n}}} - 2\].
Here, n is the number of chiral centres in the given molecule.
Complete answer:
The given structure is,
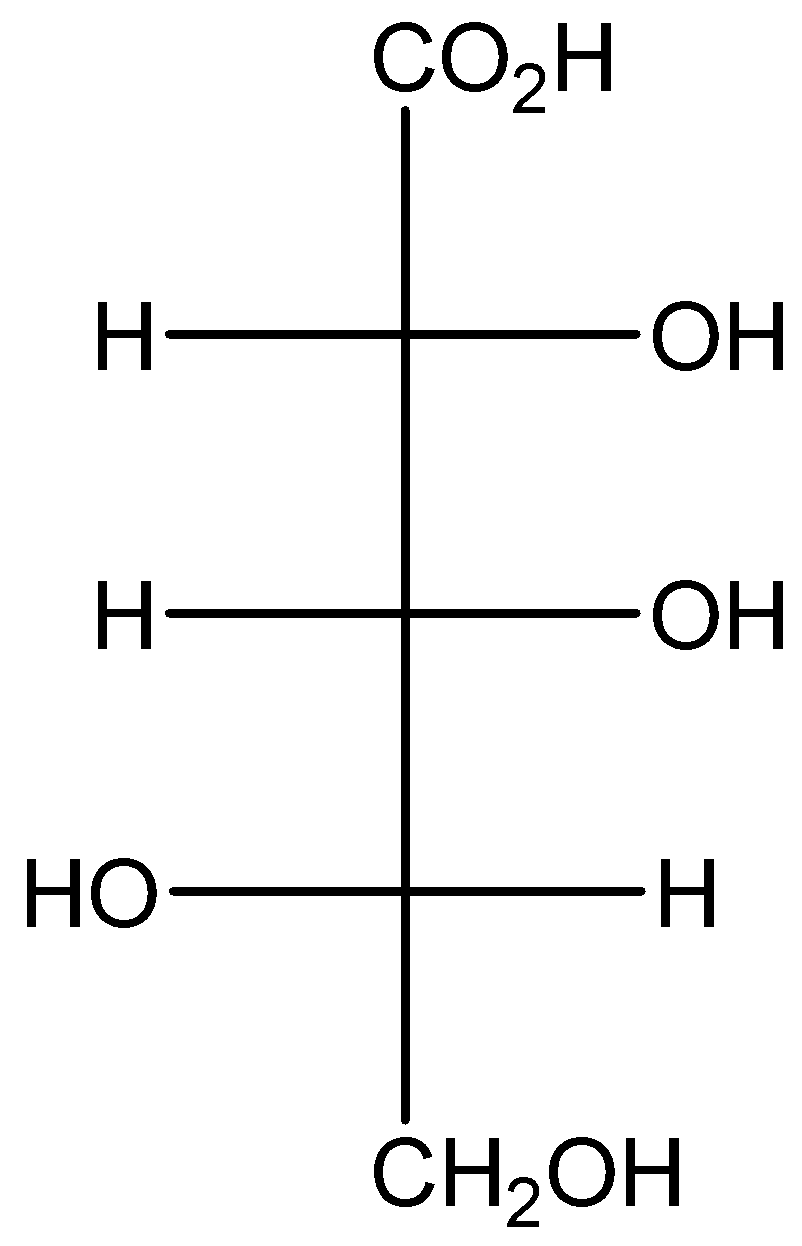
Calculate the number of optical isomer in the given structure as,
The number of optical isomers is =\[{{\text{2}}^{\text{n}}}\].
Here, n is the number of chiral centres in the given molecule.
The number of chiral carbon in given structure is mention below,
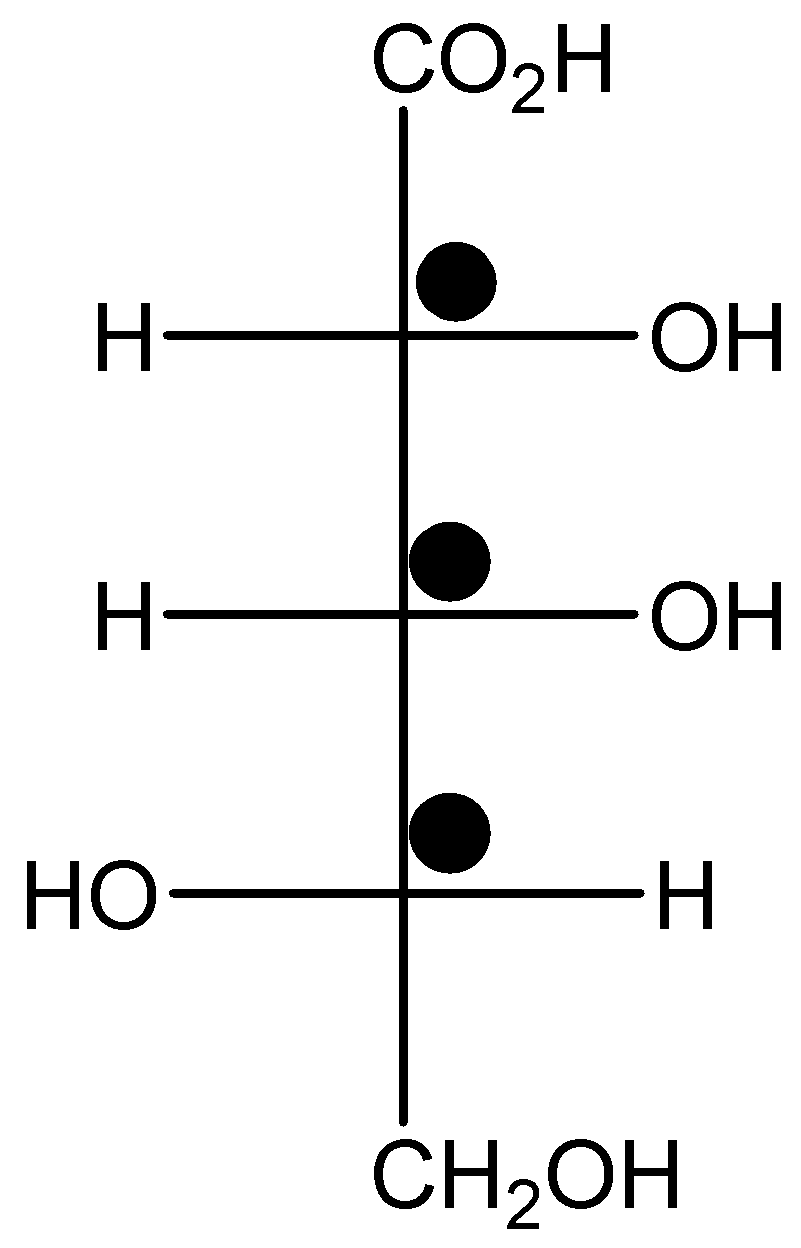
There are three chiral carbon centres in the given molecule.
\[{\text{n = 3}}\].
The number of optical isomers is =\[{{\text{2}}^{\text{n}}}\].
\[{\text{ = }}{{\text{2}}^{\text{3}}} = 8\]
There are eight optical isomers possible in the given structural molecule.
Calculate the number of diastereomers in the given structure as,
The number of diastereomers is =\[{{\text{2}}^{\text{n}}} - 2\].
\[{\text{n = 3}}\].
\[{\text{ = }}{{\text{2}}^{\text{3}}} - 2\]
\[ = 8 - 2 = 6\]
So, there are six diastereomers possible in the given structural molecule.
According to the above calculation, we conclude there are six diastereomers possible in the given structural molecule.
Hence, option C is the correct answer.
Note:
We have to know that an optical isomer should have a chiral carbon or asymmetric centre in a molecule. In organic chemistry isomers play a major role. In reaction time isomer plays an important role. Depending on the isomer of reactant only product will be obtained. In some cases equal amounts of two isomers will be obtained in the reaction. Acids and esters are called Tautomer.
Formula used:
The number of optical isomers is =\[{{\text{2}}^{\text{n}}}\].
The number of diastereomers is =\[{{\text{2}}^{\text{n}}} - 2\].
Here, n is the number of chiral centres in the given molecule.
Complete answer:
The given structure is,

Calculate the number of optical isomer in the given structure as,
The number of optical isomers is =\[{{\text{2}}^{\text{n}}}\].
Here, n is the number of chiral centres in the given molecule.
The number of chiral carbon in given structure is mention below,

There are three chiral carbon centres in the given molecule.
\[{\text{n = 3}}\].
The number of optical isomers is =\[{{\text{2}}^{\text{n}}}\].
\[{\text{ = }}{{\text{2}}^{\text{3}}} = 8\]
There are eight optical isomers possible in the given structural molecule.
Calculate the number of diastereomers in the given structure as,
The number of diastereomers is =\[{{\text{2}}^{\text{n}}} - 2\].
\[{\text{n = 3}}\].
\[{\text{ = }}{{\text{2}}^{\text{3}}} - 2\]
\[ = 8 - 2 = 6\]
So, there are six diastereomers possible in the given structural molecule.
According to the above calculation, we conclude there are six diastereomers possible in the given structural molecule.
Hence, option C is the correct answer.
Note:
We have to know that an optical isomer should have a chiral carbon or asymmetric centre in a molecule. In organic chemistry isomers play a major role. In reaction time isomer plays an important role. Depending on the isomer of reactant only product will be obtained. In some cases equal amounts of two isomers will be obtained in the reaction. Acids and esters are called Tautomer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




