
Diamond is a
A.solid containing hydrogen bonds
B.ionic solid
C.covalent solid
D.metallic solid
Answer
592.8k+ views
Hint: We must know that diamond and graphite both are the allotropes of carbon and so they have identical chemical properties. In diamond, each atom here shares the electrons with 4 other carbon atoms. Diamond is very strong in comparison to graphite as graphite exhibits a brittle and soft state. The sheets of carbon become bonded with weak covalent bonds during a tetrahedral structure. The other sheets of carbon become bonded by weak intermolecular forces. Due to the weak intermolecular forces, the layers of graphite can slide upon each other and make the substance very weak in comparison with diamonds. Also, diamond is difficult to break due to its giant covalent lattice and its many strong covalent bonds.
Complete step by step answer:
Diamond is a network covalent solid with no molecular boundaries.
Explanation:
Diamond is a naturally occurring mineral that is completely composed of only carbon atoms. Each carbon atom in a diamond is surrounded by four other carbon atoms and connected to one another by strong covalent bonds. Such an arrangement of covalently bound carbon atoms in the tetrahedral array makes the diamond structure the toughest substance.
Covalent bonds are the strongest type of chemical bond.
To break up the solid structure of a diamond, a lot of energy has to be forced to break the covalently bound strong \[C - C\] bonds. Diamond is a network solid and is a never-ending repetition of carbon atoms bonded to each other by covalent bonds.
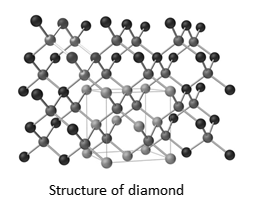
As we can see in this above diagram, a perfect single crystal of a covalent solid of diamond thus makes a single giant molecule and it is a network covalent solid with no molecular boundaries.
Thus, Diamond is a covalent solid.
Hence, option C is the correct option.
Additional information:
1.The physical properties of diamond, melting point/boiling point, are so high as to be almost immeasurable due to the endless repetition of molecules in a structure of diamond.
2. As diamond is composed of the element carbon, it is falsely believed that it must have formed from coal and this is still taught in many classrooms. But it is not true.
Note:
We must know that the covalent solids are composed of a network of or chain of atoms or molecules that are held together by covalent bonds. Metallic solids are made up of metal cations that are held together by delocalized valence electrons with metallic bonds. Ionic solids are composed of positive and negative ions that are held together by electrostatic attractions.
Complete step by step answer:
Diamond is a network covalent solid with no molecular boundaries.
Explanation:
Diamond is a naturally occurring mineral that is completely composed of only carbon atoms. Each carbon atom in a diamond is surrounded by four other carbon atoms and connected to one another by strong covalent bonds. Such an arrangement of covalently bound carbon atoms in the tetrahedral array makes the diamond structure the toughest substance.
Covalent bonds are the strongest type of chemical bond.
To break up the solid structure of a diamond, a lot of energy has to be forced to break the covalently bound strong \[C - C\] bonds. Diamond is a network solid and is a never-ending repetition of carbon atoms bonded to each other by covalent bonds.

As we can see in this above diagram, a perfect single crystal of a covalent solid of diamond thus makes a single giant molecule and it is a network covalent solid with no molecular boundaries.
Thus, Diamond is a covalent solid.
Hence, option C is the correct option.
Additional information:
1.The physical properties of diamond, melting point/boiling point, are so high as to be almost immeasurable due to the endless repetition of molecules in a structure of diamond.
2. As diamond is composed of the element carbon, it is falsely believed that it must have formed from coal and this is still taught in many classrooms. But it is not true.
Note:
We must know that the covalent solids are composed of a network of or chain of atoms or molecules that are held together by covalent bonds. Metallic solids are made up of metal cations that are held together by delocalized valence electrons with metallic bonds. Ionic solids are composed of positive and negative ions that are held together by electrostatic attractions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




