
How do you determine the number of neutrons in carbon?
Answer
561k+ views
Hint: Carbon is the element in the periodic table whose atomic number is 6. To solve this question it is important to remember the representation of the element in the periodic table and definition as well as the difference between atomic number and mass number. This gives the lead to answer this question.
Complete step by step answer:
First, let’s see the representation atom structure in the periodic table.
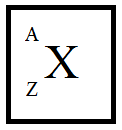
Where, X is the element
A is the mass number
Z is the atomic number
Mass number can be defined as the mass of the atom. The number of protons present in the nucleus is known as atomic number.
The relationship between A and Z is,
A= Z+ N………………………….equation 1
Where, N is the number of neutrons.
Atomic number = Number of protons = Number of electrons
Z= P= e ………………………….equation 2
Where, P is the number of protons
e is the number of electrons.
Now let's find the number of neutrons present in carbon atom
With reference to equation 2 we can conclude that the proton present in C-12 is 6.
We know, atomic mass (A) is 12.
Number of proton (P) = 6
Substituting these value in equation we can find number of neutrons present in C-13:
A = Z+ N ( Z = P)
12 = 6 + N
N = 6
Thus in carbon, the number of neutrons present is 6.
Note: Isotopes of an element can be defined as the different atomic mass exhibited by the element but have the same atomic number. As carbon has three isotopes they are: ${{C}^{12}}$, ${{C}^{13}}$, ${{C}^{14}}$. These isotopes have the same atomic number which is 6. So according to the change in atomic mass, the number of neutrons also changes. In our case that number of neutrons in carbon is asked which means C-12.
Complete step by step answer:
First, let’s see the representation atom structure in the periodic table.
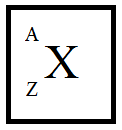
Where, X is the element
A is the mass number
Z is the atomic number
Mass number can be defined as the mass of the atom. The number of protons present in the nucleus is known as atomic number.
The relationship between A and Z is,
A= Z+ N………………………….equation 1
Where, N is the number of neutrons.
Atomic number = Number of protons = Number of electrons
Z= P= e ………………………….equation 2
Where, P is the number of protons
e is the number of electrons.
Now let's find the number of neutrons present in carbon atom
With reference to equation 2 we can conclude that the proton present in C-12 is 6.
We know, atomic mass (A) is 12.
Number of proton (P) = 6
Substituting these value in equation we can find number of neutrons present in C-13:
A = Z+ N ( Z = P)
12 = 6 + N
N = 6
Thus in carbon, the number of neutrons present is 6.
Note: Isotopes of an element can be defined as the different atomic mass exhibited by the element but have the same atomic number. As carbon has three isotopes they are: ${{C}^{12}}$, ${{C}^{13}}$, ${{C}^{14}}$. These isotopes have the same atomic number which is 6. So according to the change in atomic mass, the number of neutrons also changes. In our case that number of neutrons in carbon is asked which means C-12.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




