
How do you determine the density from a graph of volume and mass ?
Answer
552.3k+ views
Hint: Since the slope of the line through mass on the y axis and volume on the x axis.
Density is Mass concluded VolumeD=$\dfrac{M}{V}$
Slope =$\dfrac{ y}{x}$
So uncertainty you graph Mass =y and Volume = x
The Slope is the usual Density
This is a great technique of defining Density
Complete step by step answer:
A mass versus volume graph has mass, typically in grams or kilograms, on the y-axis, which is the vertical axis, and volume on the x-axis, which is the horizontal access.
You can use this graph to figure out how much mass is in any specified volume of a substance. By means of the x-axis, localize the volume you're speculating about, then find the y-intercept and use the y-axis to find how much mass there is in that much volume. For example, if we had an example of a volume of six cubic centimeters, founded on the mass versus volume graph,.
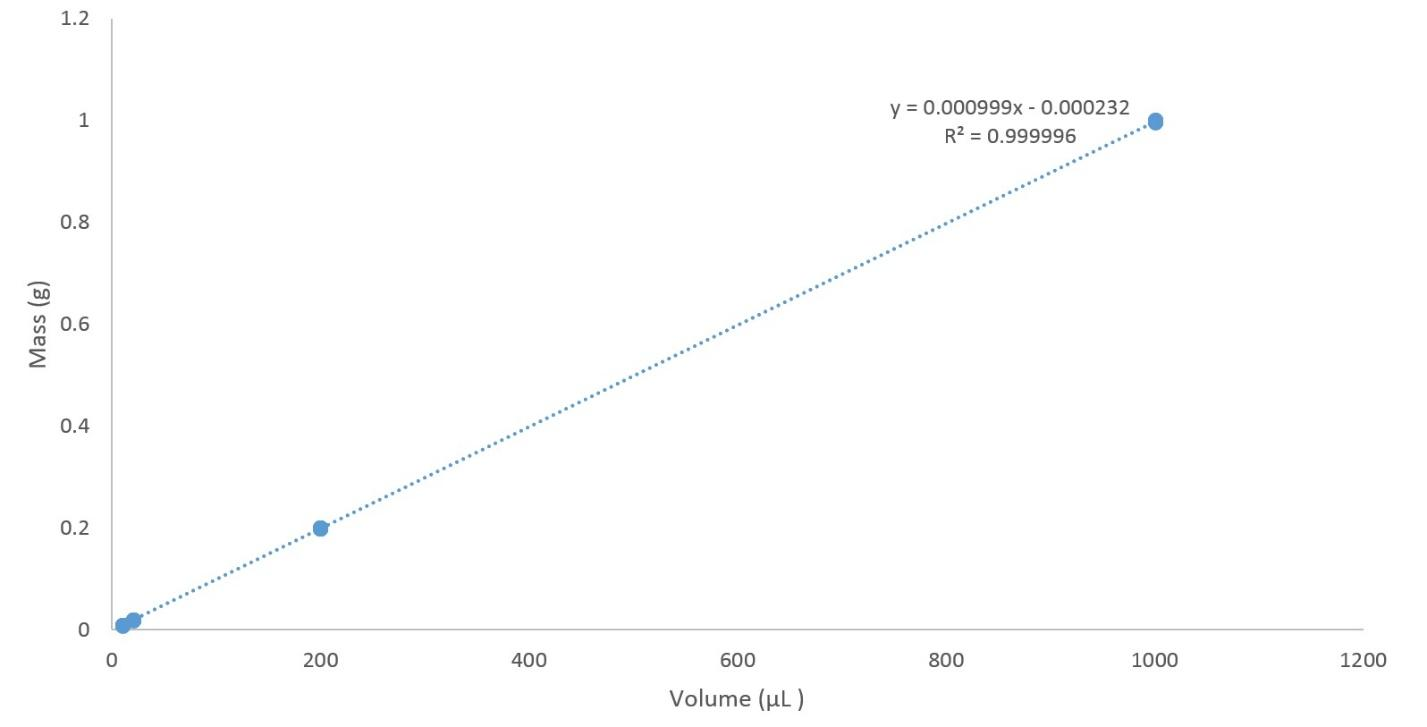
The formula meant for the slope of a straight line is the change in y divided by the change in x. Then the y axis is equal to mass, and the x axis is equal to volume, slope is equal to mass separated by volume. Then, the slope of a mass versus volume graph is equal to density.
Comparing Density
Since slope is equal to density, just glancing at a mass as against a volume graph can sometimes benefit you to recognize which of the two substances has a greater density.
Note: A steeper line specifies a greater slope and therefore a greater density. So, either substance has a steeper line or has a greater density. You can similarly practice the intentions we just discussed to invent the density of a substance.
Density is Mass concluded VolumeD=$\dfrac{M}{V}$
Slope =$\dfrac{ y}{x}$
So uncertainty you graph Mass =y and Volume = x
The Slope is the usual Density
This is a great technique of defining Density
Complete step by step answer:
A mass versus volume graph has mass, typically in grams or kilograms, on the y-axis, which is the vertical axis, and volume on the x-axis, which is the horizontal access.
You can use this graph to figure out how much mass is in any specified volume of a substance. By means of the x-axis, localize the volume you're speculating about, then find the y-intercept and use the y-axis to find how much mass there is in that much volume. For example, if we had an example of a volume of six cubic centimeters, founded on the mass versus volume graph,.

The formula meant for the slope of a straight line is the change in y divided by the change in x. Then the y axis is equal to mass, and the x axis is equal to volume, slope is equal to mass separated by volume. Then, the slope of a mass versus volume graph is equal to density.
Comparing Density
Since slope is equal to density, just glancing at a mass as against a volume graph can sometimes benefit you to recognize which of the two substances has a greater density.
Note: A steeper line specifies a greater slope and therefore a greater density. So, either substance has a steeper line or has a greater density. You can similarly practice the intentions we just discussed to invent the density of a substance.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




