
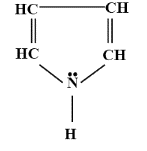
How many delocalised $\pi $-electrons are there in the following compound ?
A. 1
B. 2
C. 4
D. 6

Answer
599.7k+ views
Hint: A pair of electrons which do not make any bond are known as lone pairs whereas a pair of electrons, which is found in the paired condition they are known as electron pairs. Lone pairs electrons can move in the lattice structure.
Complete step by step answer:
- The delocalised electrons are those electrons which can move from one bond to the other in a molecule structure.
- The structure which is given in the question is of pyrroles, a 5-membered ring.
- In the given, we have to tell about the delocalised electron but they should belong to $\pi $- electrons.
- $\pi $-electrons are those electrons which are formed when a double-bond is formed i.e. they are found in the double-bond.
- So, as we can see that there are a total of two double bonds so there are 4 $\pi $-electrons.
- Along with it, two delocalised $\pi $-electrons are also present above the nitrogen atom.
- So, a total of 6 delocalised $\pi $- electrons are present in the pyrrole.
- These delocalised electrons are also responsible for the electrical conduction due to their movement in the lattice structure.
Therefore, option D. is the correct answer.
Note: $\pi $-electrons are found in the double bond whereas $\sigma $- electrons are found in the single bond. Usually without the formation of sigma bond the formation of $\pi $bond can not take place. Moreover, benzene is another example of the delocalised $\pi $-electron due to which the benzene has a stable structure.
Complete step by step answer:
- The delocalised electrons are those electrons which can move from one bond to the other in a molecule structure.
- The structure which is given in the question is of pyrroles, a 5-membered ring.
- In the given, we have to tell about the delocalised electron but they should belong to $\pi $- electrons.
- $\pi $-electrons are those electrons which are formed when a double-bond is formed i.e. they are found in the double-bond.
- So, as we can see that there are a total of two double bonds so there are 4 $\pi $-electrons.
- Along with it, two delocalised $\pi $-electrons are also present above the nitrogen atom.
- So, a total of 6 delocalised $\pi $- electrons are present in the pyrrole.
- These delocalised electrons are also responsible for the electrical conduction due to their movement in the lattice structure.
Therefore, option D. is the correct answer.
Note: $\pi $-electrons are found in the double bond whereas $\sigma $- electrons are found in the single bond. Usually without the formation of sigma bond the formation of $\pi $bond can not take place. Moreover, benzene is another example of the delocalised $\pi $-electron due to which the benzene has a stable structure.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




