
Define aufbau principle.
Answer
588k+ views
Hint: There are certain rules according to which electrons are filled in an atom and there are some principles that explain these rules. Afbau principle is among those principles. This principle explains the filling of electrons in different shells of an atom. There are different electrons in different shells of an atom.
Complete step by step answer:
As we know there are certain rules which are to be followed while filling electrons in an atom. Each atom has different shells. These shells have subshells and subshells contain different orbitals. According to aufbau principle before occupying higher orbitals lower energy orbitals must be filled. According to this principle orbital having lowest energy is filled first. Then higher energy orbital is filled and so on. To be more specific aufbau principle states that in the ground state of an ion, electrons fill atomic orbitals of lowest available energy before occupying higher levels. According to this principle order in which orbitals are filled is:
$\begin{array}{*{20}{c}}
{1s}&{2s}&{2p}&{3s}&{3p}&{4s}&{3d}&{4p}&{5s}&{4d\begin{array}{*{20}{c}}
.&.&.
\end{array}}
\end{array}$
This series can be remembered as:
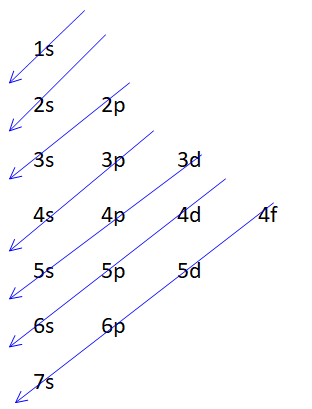
Orbital which is being cut by the diagonal arrow first is filled first then other and so on. Maximum number of electrons in a shell can be $2{n^2}$. Where $n$ is the number of shells that is the principal quantum number. Maximum number of electrons in a subshell is equal to $2\left( {2l + 1} \right)$ where $l$ is an angular quantum number.
Note:
Each atom contains some shells. Each shell has some subshells. These subshells are $s,p,d,f$. Each subshell contains some orbitals. $s$ subshell has one orbital, $p$ subshell has three orbitals, $d$ subshell has five orbitals and $d$ subshell has seven orbitals. In each orbital maximum of two electrons can be filled.
Complete step by step answer:
As we know there are certain rules which are to be followed while filling electrons in an atom. Each atom has different shells. These shells have subshells and subshells contain different orbitals. According to aufbau principle before occupying higher orbitals lower energy orbitals must be filled. According to this principle orbital having lowest energy is filled first. Then higher energy orbital is filled and so on. To be more specific aufbau principle states that in the ground state of an ion, electrons fill atomic orbitals of lowest available energy before occupying higher levels. According to this principle order in which orbitals are filled is:
$\begin{array}{*{20}{c}}
{1s}&{2s}&{2p}&{3s}&{3p}&{4s}&{3d}&{4p}&{5s}&{4d\begin{array}{*{20}{c}}
.&.&.
\end{array}}
\end{array}$
This series can be remembered as:

Orbital which is being cut by the diagonal arrow first is filled first then other and so on. Maximum number of electrons in a shell can be $2{n^2}$. Where $n$ is the number of shells that is the principal quantum number. Maximum number of electrons in a subshell is equal to $2\left( {2l + 1} \right)$ where $l$ is an angular quantum number.
Note:
Each atom contains some shells. Each shell has some subshells. These subshells are $s,p,d,f$. Each subshell contains some orbitals. $s$ subshell has one orbital, $p$ subshell has three orbitals, $d$ subshell has five orbitals and $d$ subshell has seven orbitals. In each orbital maximum of two electrons can be filled.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




