
$Cr - C$ bond in the compound $[Cr{(CO)_6}]$ shows $\pi - $ character due to :
A. covalent bonding
B. coordinate bonding
C. synergic bonding
D. ionic bonding
Answer
598.8k+ views
Hint: We have studied in complex compound that metal carbonyls are coordination complexes of transition metals where carbon monoxide ($CO$) is acts as ligand (ligands are ion, atom or molecule that bonds to central metal, it involves in donation of one or more of its electrons). The important point to mention here is that $CO$ is a neutral and strong field ligand which tends to pairing of electrons. This complex compound is diamagnetic in nature because of strong field ligands. It is ${d^2}s{p^3}$ hybridized.
Complete answer:
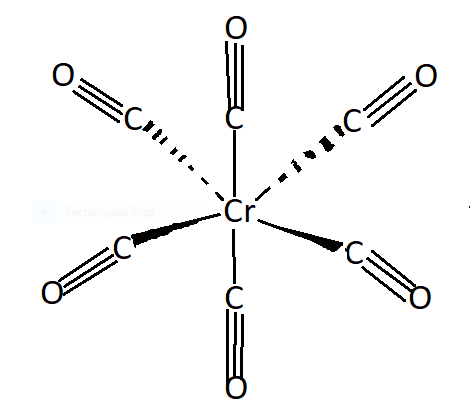
See the following structure of chromium hexacarbonyl in the figure. $CO$ is a $\pi $ - acceptor ligand ,to form a metal centre it accepts electron cloud density which results in synergistic effect. $Cr - C$ is stronger whereas the $C - O$ bond is weaker. Chromium metal receives electron density via $\sigma $-bond and chromium donates back via pi- bond. So in synergic bonding electrons are transferred from ligands to metal. It is bonding between carbonyl groups (ligand) and metal . It is a self strengthening bond. So $Cr - C$ bond in the compound $[Cr{(CO)_6}]$ shows $\pi - $ character due to synergic bonding.
Note: Now we know that Synergic bonding can be named as back-bonding. Due to this bonding carbonyl group donates its pair of electrons to an empty d orbitals, so due to this kind of bonding
$Cr - C$ bond in the compound $[Cr{(CO)_6}]$ shows $\pi - $ character. We also get some information about the coordinate compound chromium hexacarbonyl. Chromium carbonyl is known as chromium hexacarbonyl. In this complex compound Cr has zero oxidation state .It is a colourless crystal. The coordination geometry of the complex is octahedral.
Complete answer:
See the following structure of chromium hexacarbonyl in the figure. $CO$ is a $\pi $ - acceptor ligand ,to form a metal centre it accepts electron cloud density which results in synergistic effect. $Cr - C$ is stronger whereas the $C - O$ bond is weaker. Chromium metal receives electron density via $\sigma $-bond and chromium donates back via pi- bond. So in synergic bonding electrons are transferred from ligands to metal. It is bonding between carbonyl groups (ligand) and metal . It is a self strengthening bond. So $Cr - C$ bond in the compound $[Cr{(CO)_6}]$ shows $\pi - $ character due to synergic bonding.

Note: Now we know that Synergic bonding can be named as back-bonding. Due to this bonding carbonyl group donates its pair of electrons to an empty d orbitals, so due to this kind of bonding
$Cr - C$ bond in the compound $[Cr{(CO)_6}]$ shows $\pi - $ character. We also get some information about the coordinate compound chromium hexacarbonyl. Chromium carbonyl is known as chromium hexacarbonyl. In this complex compound Cr has zero oxidation state .It is a colourless crystal. The coordination geometry of the complex is octahedral.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




