
How many covalent bonds are there in a molecule of ethane $\left( {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}} \right)$?
Answer
606.3k+ views
Hint: Once we know about the structure of ethane, we will have a clear idea about the bonding it possesses. Then we can figure out the number of covalent bonds present in a molecule of ethane.
Complete step-by-step answer:
The structure of ethane is given below:
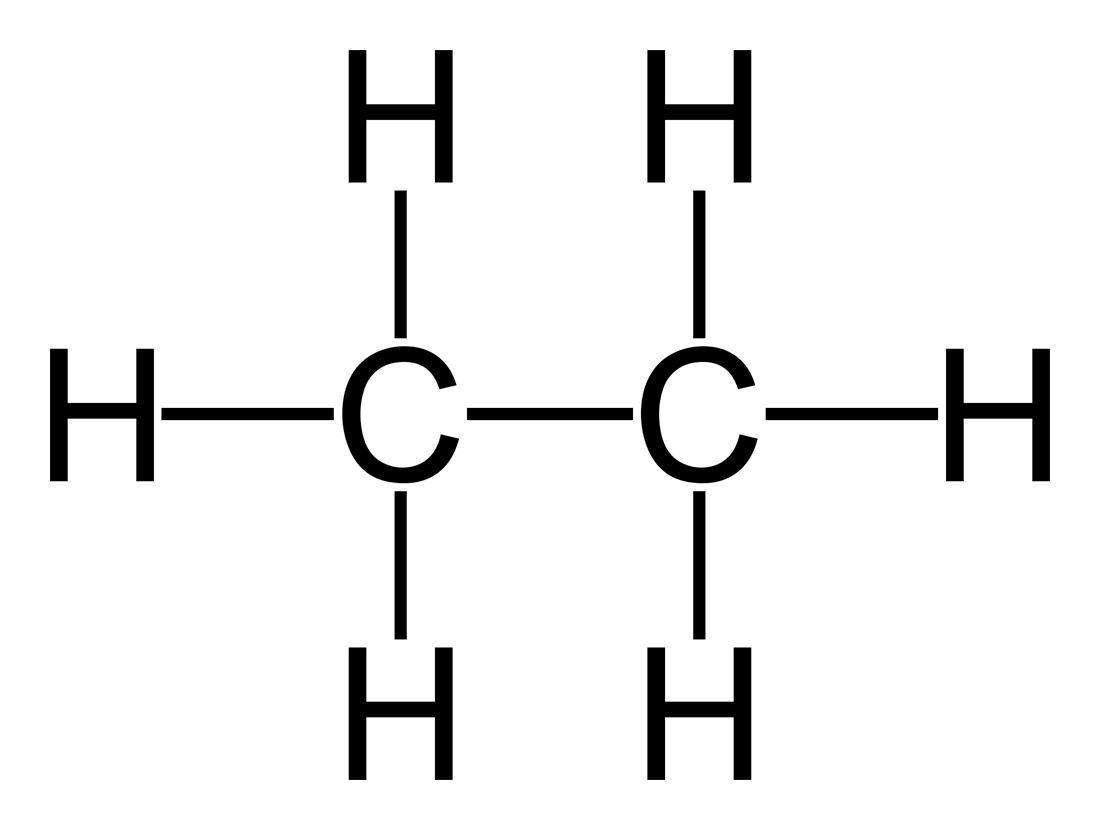
As we know that there are 2 carbon atoms and there are 6 hydrogen atoms present in the compound. By looking at this chemical structure the two carbon atoms are bonded together and there are three hydrogen atoms attached to each carbon atom of the bond that we see here a single bond for this reason it is classified as an alkane.
Therefore, we can see that there are 7 covalent bonds formed. So, the answer to the question of how many covalent bonds are there in one molecule of ethane is 7.
Note: Some more information of ethane is that it is colourless at room temperature. It has a slightly sweet smell and it is flammable.
Ethane is used in the production of Ethylene for making plastics, antifreeze and detergents, it’s also a ripening agent for fruits, a refrigerant, a substance introducing welding gas and the primary ingredient in mustard gas. Ethane is a component in the natural gas Methane and is removed by cryogenic liquefaction.
Ethane is the simplest hydrocarbon containing more than one carbon atom. It is a prominent compound of industrial importance by converting it to ethylene or using it as a building block for the petrochemical industry, for the synthesis of both polyethylene plastic and other small compounds.
Complete step-by-step answer:
The structure of ethane is given below:

As we know that there are 2 carbon atoms and there are 6 hydrogen atoms present in the compound. By looking at this chemical structure the two carbon atoms are bonded together and there are three hydrogen atoms attached to each carbon atom of the bond that we see here a single bond for this reason it is classified as an alkane.
Therefore, we can see that there are 7 covalent bonds formed. So, the answer to the question of how many covalent bonds are there in one molecule of ethane is 7.
Note: Some more information of ethane is that it is colourless at room temperature. It has a slightly sweet smell and it is flammable.
Ethane is used in the production of Ethylene for making plastics, antifreeze and detergents, it’s also a ripening agent for fruits, a refrigerant, a substance introducing welding gas and the primary ingredient in mustard gas. Ethane is a component in the natural gas Methane and is removed by cryogenic liquefaction.
Ethane is the simplest hydrocarbon containing more than one carbon atom. It is a prominent compound of industrial importance by converting it to ethylene or using it as a building block for the petrochemical industry, for the synthesis of both polyethylene plastic and other small compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




