
Correct relationship between pairing energy (P) and C.F.S.E $\left( {{{{\Delta }}_{\text{0}}}} \right)$ in complex ion ${\left[ {Ir{{\left( {{H_2}O} \right)}_6}} \right]^{3 + }}$ is:
A. ${{{\Delta }}_{\text{0}}}{\text{ < P}}$
B. ${{{\Delta }}_{\text{0}}}{\text{ > P}}$
C. ${{{\Delta }}_{\text{0}}}{\text{ = P}}$
D. Cannot comment
Answer
582.3k+ views
Hint: We can say that the energy penalty for placing two electrons in the same orbital, ensuing from the electrostatic repulsion between electrons is called Pairing energy (P).
Complete step by step answer:
We remember that the d-orbitals of the central metal atom are divided into two sets of different energies when ligand approaches. The separation in energy is the crystal field splitting energy $\left( {\text{\Delta }} \right)$ when splitting energy is large it is more favorable for electrons to occupy the lower set of orbitals (strong ligands) when the splitting energy is small it is energetically more favorable for the electrons to occupy both the sets with parallel electrons spins as possible (weak ligands).
We can define Crystal Field Stabilization Energy as the energy difference of the electron configuration in the ligand field to the energy of the electronic configuration in the isotropic field. We can write the expression for the crystal field stabilization energy as,
${\text{CFSE}}\left( {\Delta E} \right) = {{\text{E}}_{{\text{ligand field}}}} - {{\text{E}}_{{\text{isotropic field}}}}$
We have to know that Crystal Field Stabilization Energy depends on factors like number of d-electrons, ligand character, geometry, and spin pairing energy.
We use the term spin pairing energy P, if the electrons are paired inside a single orbital.
We can write the spectrochemical series as,
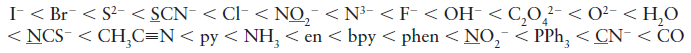
If ${{{\Delta }}_{\text{o}}}{\text{ > P}}$, then the complex would be low spin and if ${{{\Delta }}_{\text{o}}}{\text{ < P}}$, the compound would be high spin.
Because \[{{{\Delta }}_{\text{O}}}\] is dependent on both the metals and the ligands, we can use it to determine the spin state of the complex.
We can say a strong ligand or a strong field ligand is a ligand that can result in a higher crystal field splitting.
We can say a weak ligand or a weak field ligand is a ligand that can result in a lower crystal field splitting.
In the spectrochemical series, we have to know that ligands up to water are weak-field ligands and have a tendency to result in high-spin complexes.
In the spectrochemical series, we have to know that ligands beyond water are strong-field ligands and have a tendency to result in low-spin complexes.
We know that water is a weak field ligand. Thus, the crystal field splitting could be small and therefore, ${{{\Delta }}_{\text{0}}}{\text{ < P}}$.
So, the correct answer is Option A .
Note:
The binding of a strong field ligand causes a higher difference between the higher and lower energy level orbitals.
Examples: \[{\text{C}}{{\text{N}}^{\text{-}}}\](cyanide ligands), \[{\text{N}}{{\text{O}}_{\text{2}}}^{\text{-}}\](nitro ligand) and \[{\text{CO}}\](carbonyl ligands)
The binding of a weak field ligand causes a lower difference between the higher and lower energy level orbitals since the low difference between the two orbital levels causes repulsions between electrons in those energy levels, the higher energy orbitals can be easily filled with electrons when compared to that in low energy orbitals.
Examples: \[{{\text{I}}^{\text{-}}}\](iodide ligand), \[{\text{B}}{{\text{r}}^{\text{-}}}\](bromide ligand), etc
Complete step by step answer:
We remember that the d-orbitals of the central metal atom are divided into two sets of different energies when ligand approaches. The separation in energy is the crystal field splitting energy $\left( {\text{\Delta }} \right)$ when splitting energy is large it is more favorable for electrons to occupy the lower set of orbitals (strong ligands) when the splitting energy is small it is energetically more favorable for the electrons to occupy both the sets with parallel electrons spins as possible (weak ligands).
We can define Crystal Field Stabilization Energy as the energy difference of the electron configuration in the ligand field to the energy of the electronic configuration in the isotropic field. We can write the expression for the crystal field stabilization energy as,
${\text{CFSE}}\left( {\Delta E} \right) = {{\text{E}}_{{\text{ligand field}}}} - {{\text{E}}_{{\text{isotropic field}}}}$
We have to know that Crystal Field Stabilization Energy depends on factors like number of d-electrons, ligand character, geometry, and spin pairing energy.
We use the term spin pairing energy P, if the electrons are paired inside a single orbital.
We can write the spectrochemical series as,
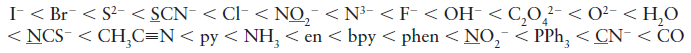
If ${{{\Delta }}_{\text{o}}}{\text{ > P}}$, then the complex would be low spin and if ${{{\Delta }}_{\text{o}}}{\text{ < P}}$, the compound would be high spin.
Because \[{{{\Delta }}_{\text{O}}}\] is dependent on both the metals and the ligands, we can use it to determine the spin state of the complex.
We can say a strong ligand or a strong field ligand is a ligand that can result in a higher crystal field splitting.
We can say a weak ligand or a weak field ligand is a ligand that can result in a lower crystal field splitting.
In the spectrochemical series, we have to know that ligands up to water are weak-field ligands and have a tendency to result in high-spin complexes.
In the spectrochemical series, we have to know that ligands beyond water are strong-field ligands and have a tendency to result in low-spin complexes.
We know that water is a weak field ligand. Thus, the crystal field splitting could be small and therefore, ${{{\Delta }}_{\text{0}}}{\text{ < P}}$.
So, the correct answer is Option A .
Note:
The binding of a strong field ligand causes a higher difference between the higher and lower energy level orbitals.
Examples: \[{\text{C}}{{\text{N}}^{\text{-}}}\](cyanide ligands), \[{\text{N}}{{\text{O}}_{\text{2}}}^{\text{-}}\](nitro ligand) and \[{\text{CO}}\](carbonyl ligands)
The binding of a weak field ligand causes a lower difference between the higher and lower energy level orbitals since the low difference between the two orbital levels causes repulsions between electrons in those energy levels, the higher energy orbitals can be easily filled with electrons when compared to that in low energy orbitals.
Examples: \[{{\text{I}}^{\text{-}}}\](iodide ligand), \[{\text{B}}{{\text{r}}^{\text{-}}}\](bromide ligand), etc
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




