
What is the correct dipole moment of $N{{H}_{3}}$ and $N{{F}_{3}}$ respectively?
(A)- $4.90\times {{10}^{-30}}$ C m and $0.80\times {{10}^{-30}}$ C m
(B)- $0.80\times {{10}^{-30}}$ C m and $4.90\times {{10}^{-30}}$ C m
(C)- $4.90\times {{10}^{-30}}$ C m and $4.90\times {{10}^{-30}}$ C m
(D)- $0.80\times {{10}^{-30}}$ C m and $0.80\times {{10}^{-30}}$ C m
Answer
593.1k+ views
Hint: Dipole moment is a measure of polarity of a bond. It is the product of the charges and the distance between partial charges. It is a vector quantity and its direction is always given from less electronegative atom to more electronegative atom.
It is generally expressed in debye (D) and 1 D = $3.33564\times {{10}^{-30}}$ C m.
Dipole moment of polar molecules containing lone pairs is the vector sum of dipole of lone pair and net dipole moments of bonds.
Complete answer:
Both $N{{H}_{3}}$ and $N{{F}_{3}}$ have trigonal pyramidal shape.
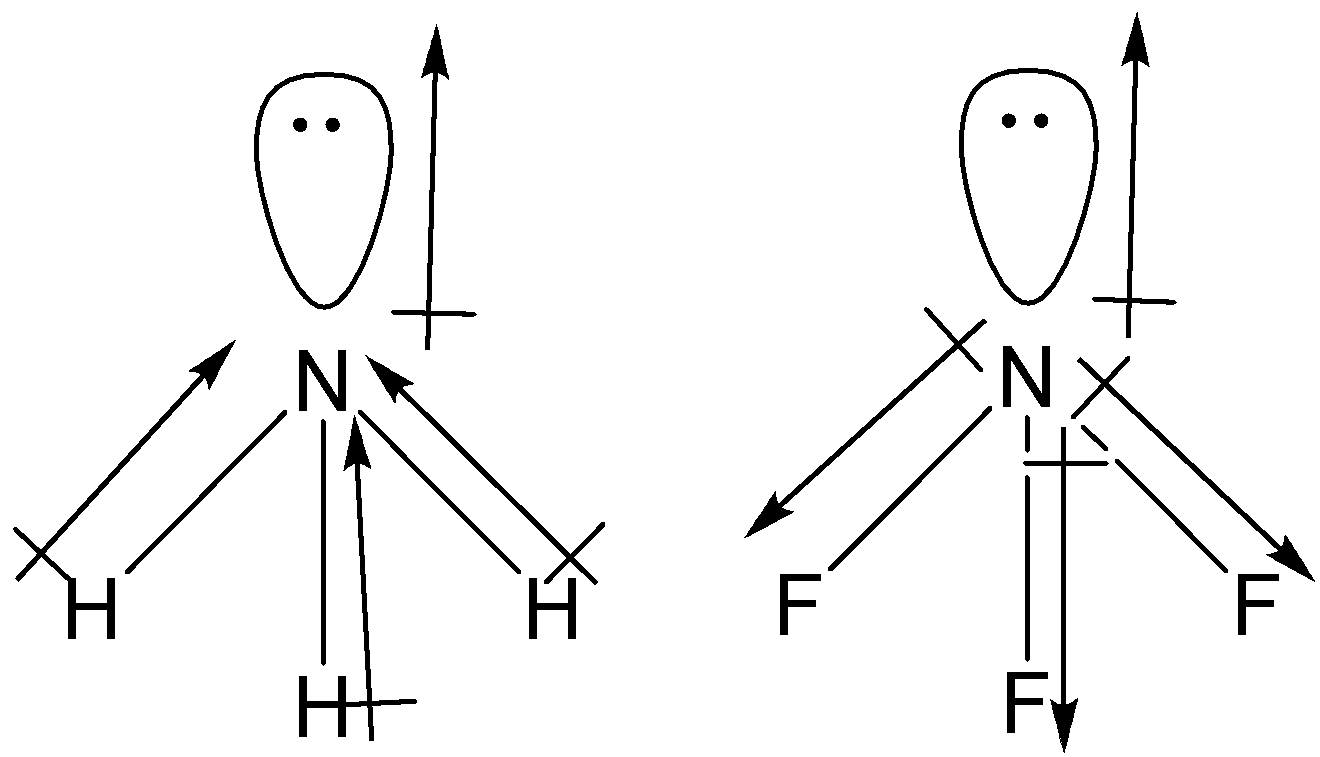
The dipole moment of lone pairs in $N{{H}_{3}}$ and $N{{F}_{3}}$ is away from nitrogen.
Dipole moment of $N{{H}_{3}}$
We know that nitrogen is more electronegative than hydrogen. Therefore, the dipole moment of $N-H$bond will be form H to N. The net dipole moment of three $N-H$ bond will add up to 1.4 D. As we know that 1 D = $3.33564\times {{10}^{-34}}$ C m.
Then, 1.4 D will be equal to $1.4\times 3.33564\times {{10}^{-30}}$ C m, i.e. $4.90\times {{10}^{-30}}$ C m.
Dipole moment of $N{{F}_{3}}$
Electronegativity of F is more than that of N, thus the direction of dipole moment of $N-F$ bond will be from F to N. As we can see that the direction of $N-F$ bond is opposite to that of the lone pair on N atoms. So, the net dipole moment of $N{{F}_{3}}$ has been found to be 0.24 D.
Multiplying 0.24 D with $3.33564\times {{10}^{-30}}$ C m, we get the dipole moment of $0.80\times {{10}^{-30}}$C m.
So, the correct answer is “Option A”.
Note: The dipole moment of lone pairs in $N{{H}_{3}}$ and $N{{F}_{3}}$ is away from nitrogen.
Dipole moment of $N{{H}_{3}}$
We know that nitrogen is more electronegative than hydrogen. Therefore, the dipole moment of $N-H$bond will be form H to N. The net dipole moment of three $N-H$ bond will add up to 1.4 D. As we know that 1 D = $3.33564\times {{10}^{-34}}$ C m.
Then, 1.4 D will be equal to $1.4\times 3.33564\times {{10}^{-30}}$ C m, i.e. $4.90\times {{10}^{-30}}$ C m.
Dipole moment of $N{{F}_{3}}$
Electronegativity of F is more than that of N, thus the direction of dipole moment of $N-F$ bond will be from F to N. As we can see that the direction of $N-F$ bond is opposite to that of the lone pair on N atoms. So, the net dipole moment of $N{{F}_{3}}$ has been found to be 0.24 D.
Multiplying 0.24 D with $3.33564\times {{10}^{-30}}$ C m, we get the dipole moment of $0.80\times {{10}^{-30}}$C m.
It is generally expressed in debye (D) and 1 D = $3.33564\times {{10}^{-30}}$ C m.
Dipole moment of polar molecules containing lone pairs is the vector sum of dipole of lone pair and net dipole moments of bonds.
Complete answer:
Both $N{{H}_{3}}$ and $N{{F}_{3}}$ have trigonal pyramidal shape.

The dipole moment of lone pairs in $N{{H}_{3}}$ and $N{{F}_{3}}$ is away from nitrogen.
Dipole moment of $N{{H}_{3}}$
We know that nitrogen is more electronegative than hydrogen. Therefore, the dipole moment of $N-H$bond will be form H to N. The net dipole moment of three $N-H$ bond will add up to 1.4 D. As we know that 1 D = $3.33564\times {{10}^{-34}}$ C m.
Then, 1.4 D will be equal to $1.4\times 3.33564\times {{10}^{-30}}$ C m, i.e. $4.90\times {{10}^{-30}}$ C m.
Dipole moment of $N{{F}_{3}}$
Electronegativity of F is more than that of N, thus the direction of dipole moment of $N-F$ bond will be from F to N. As we can see that the direction of $N-F$ bond is opposite to that of the lone pair on N atoms. So, the net dipole moment of $N{{F}_{3}}$ has been found to be 0.24 D.
Multiplying 0.24 D with $3.33564\times {{10}^{-30}}$ C m, we get the dipole moment of $0.80\times {{10}^{-30}}$C m.
So, the correct answer is “Option A”.
Note: The dipole moment of lone pairs in $N{{H}_{3}}$ and $N{{F}_{3}}$ is away from nitrogen.
Dipole moment of $N{{H}_{3}}$
We know that nitrogen is more electronegative than hydrogen. Therefore, the dipole moment of $N-H$bond will be form H to N. The net dipole moment of three $N-H$ bond will add up to 1.4 D. As we know that 1 D = $3.33564\times {{10}^{-34}}$ C m.
Then, 1.4 D will be equal to $1.4\times 3.33564\times {{10}^{-30}}$ C m, i.e. $4.90\times {{10}^{-30}}$ C m.
Dipole moment of $N{{F}_{3}}$
Electronegativity of F is more than that of N, thus the direction of dipole moment of $N-F$ bond will be from F to N. As we can see that the direction of $N-F$ bond is opposite to that of the lone pair on N atoms. So, the net dipole moment of $N{{F}_{3}}$ has been found to be 0.24 D.
Multiplying 0.24 D with $3.33564\times {{10}^{-30}}$ C m, we get the dipole moment of $0.80\times {{10}^{-30}}$C m.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




