
Convert the following: Benzoic acid to Aniline
Answer
528.8k+ views
Hint: We will need to make the carboxylic acid group more reactive by converting it into some group that will easily react with other compounds that will introduce nitrogen into the molecule. We can use Hoffman bromamide degradation reaction to obtain amines from corresponding amides with the loss of one carbon.
Complete answer:
To convert Benzoic acid to Aniline, we need to remove the -COOH group from the benzene ring and need \[ - N{H_2}\] group attached to that carbon in the benzene ring. Now, any direct reaction that converts the -COOH group to \[ - N{H_2}\] group is not available. So, we will need to include more than one step in the reaction.
- We will need to convert the -COOH group into a kind of group that will help to obtain \[ - N{H_2}\] there. To get nitrogen atoms in the organic compound, we can use \[N{H_3}\] that will react with suitable functional groups. So, if we convert the -COOH group to -COCl then this acid chloride group can react with \[N{H_3}\] to give nitrogen atoms in the compound. Then, we will obtain amide as a product and hoffman bromamide degradation of this amide will give amine with one carbon atom less and that will be aniline. Let’s see the reaction.
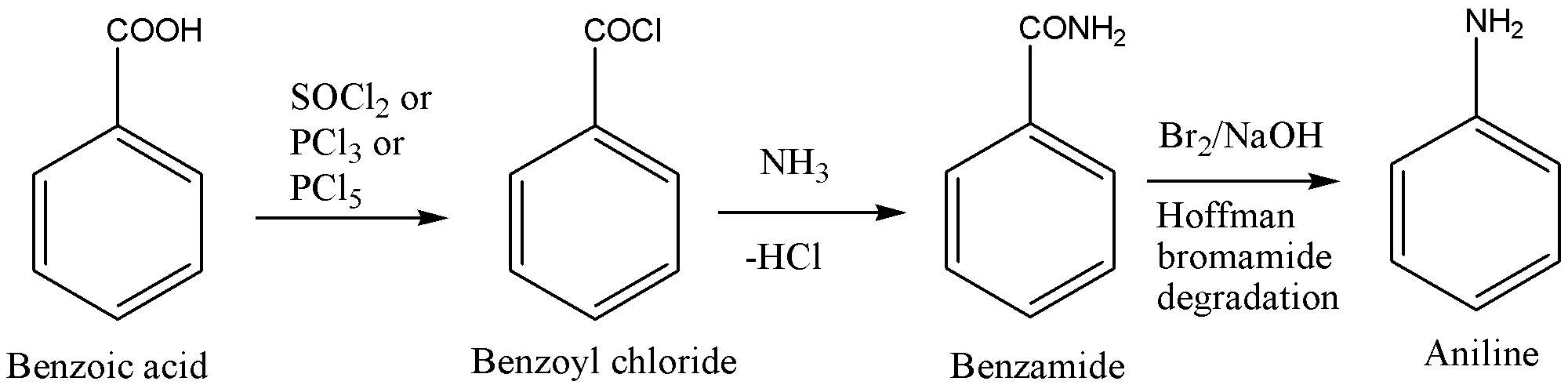
- To convert -COOH group into -COCl group, we can use Thionyl chloride, Phosphorus trichloride or Phosphorus pentachloride that will replace -OH group with -Cl and will convert Benzoic acid into Benzoyl chloride.
- Now, to add nitrogen atoms in the compound, we can allow the acid chloride to react with ammonia gas that will give corresponding amide and hydrochloric acid.
- Now conversion of benzamide to Aniline may look a bit tough but it is possible by Hoffman bromamide degradation. If we react benzamide with Bromine in presence of sodium hydroxide, then nitrogen will be first brominated and then rearrangement will occur and isocyanate will be produced, that upon hydrolysis will give an amine with one carbon less. So, we will obtain Aniline as a final product.
Note: Organic conversions may have more than one path to obtain the final product. We should choose a path that involves the reactions that are being taught to us. Remember that as direct conversion of reactant to product is not possible, do not panic that you will not be able to solve the problem. Try to think about what kind of atoms or functional groups you want and then try some reactions on them to obtain the final product.
Complete answer:
To convert Benzoic acid to Aniline, we need to remove the -COOH group from the benzene ring and need \[ - N{H_2}\] group attached to that carbon in the benzene ring. Now, any direct reaction that converts the -COOH group to \[ - N{H_2}\] group is not available. So, we will need to include more than one step in the reaction.
- We will need to convert the -COOH group into a kind of group that will help to obtain \[ - N{H_2}\] there. To get nitrogen atoms in the organic compound, we can use \[N{H_3}\] that will react with suitable functional groups. So, if we convert the -COOH group to -COCl then this acid chloride group can react with \[N{H_3}\] to give nitrogen atoms in the compound. Then, we will obtain amide as a product and hoffman bromamide degradation of this amide will give amine with one carbon atom less and that will be aniline. Let’s see the reaction.

- To convert -COOH group into -COCl group, we can use Thionyl chloride, Phosphorus trichloride or Phosphorus pentachloride that will replace -OH group with -Cl and will convert Benzoic acid into Benzoyl chloride.
- Now, to add nitrogen atoms in the compound, we can allow the acid chloride to react with ammonia gas that will give corresponding amide and hydrochloric acid.
- Now conversion of benzamide to Aniline may look a bit tough but it is possible by Hoffman bromamide degradation. If we react benzamide with Bromine in presence of sodium hydroxide, then nitrogen will be first brominated and then rearrangement will occur and isocyanate will be produced, that upon hydrolysis will give an amine with one carbon less. So, we will obtain Aniline as a final product.
Note: Organic conversions may have more than one path to obtain the final product. We should choose a path that involves the reactions that are being taught to us. Remember that as direct conversion of reactant to product is not possible, do not panic that you will not be able to solve the problem. Try to think about what kind of atoms or functional groups you want and then try some reactions on them to obtain the final product.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




