
How can we convert $ R-2-bromopentane $ to Fischer projection?
Answer
531.6k+ views
Hint :Fischer projection is defined as the two dimensional representation of the three dimensional molecule of the organic compound with help of the projection. They help in representing the monosaccharides in biochemistry and organic chemistry. It helps in depicting the stereo formula in two dimensional forms.
Complete Step By Step Answer:
So to know how the Fischer projection of a compound is formed let us take the example of $ R-2-bromopentane $ . In making the Fischer projection we should firstly draw the chain vertically and place the aldehyde group at the top. The bonds present above and below any of the two adjacent carbon atoms are present behind the plane of paper.
Whereas the horizontal bonds are coming out of paper. As we know that the geometry of carbon is tetrahedral that means the vertical groups one and four should be present behind the paper. When we have a longer vertical chain we look at any two of the adjacent atoms. The steps for conversion are:
Step1: Draw the bond-line structure of bromopentane and then convert this to a wedge-dash structure. Start by making the $ Br $ bond a wedge and the $ H $ bond a dash. We have a $ 50\text{ }% $ chance of getting it right.
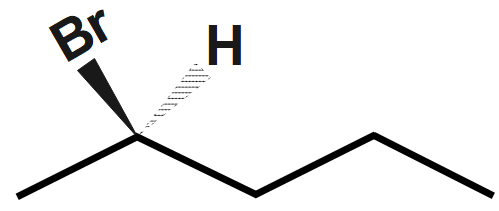
Step 2. Draw the skeleton Fischer projection
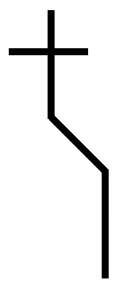
Step3: Attach the $ H $ and the $ Br $ atoms to the correct sides of the cross. We must look at the molecule from the upper right. This puts $ Br $ on the left and $ H $ on the right.
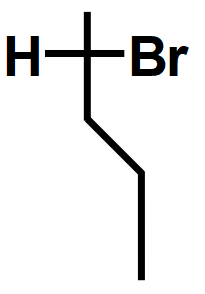
Note :
A dextrorotatory compound is prefixed with $ \left( + \right) $ or d. whereas the levorotatory compound is prefixed with the $ \left( - \right) $ or l. These lowercase are considered to be different from those of the D and L prefixes which are used in differentiating chiral configurations with the organic compounds. The carbohydrate is a biomolecule which comprises the carbon, hydrogen and the oxygen atoms.
Complete Step By Step Answer:
So to know how the Fischer projection of a compound is formed let us take the example of $ R-2-bromopentane $ . In making the Fischer projection we should firstly draw the chain vertically and place the aldehyde group at the top. The bonds present above and below any of the two adjacent carbon atoms are present behind the plane of paper.
Whereas the horizontal bonds are coming out of paper. As we know that the geometry of carbon is tetrahedral that means the vertical groups one and four should be present behind the paper. When we have a longer vertical chain we look at any two of the adjacent atoms. The steps for conversion are:
Step1: Draw the bond-line structure of bromopentane and then convert this to a wedge-dash structure. Start by making the $ Br $ bond a wedge and the $ H $ bond a dash. We have a $ 50\text{ }% $ chance of getting it right.

Step 2. Draw the skeleton Fischer projection

Step3: Attach the $ H $ and the $ Br $ atoms to the correct sides of the cross. We must look at the molecule from the upper right. This puts $ Br $ on the left and $ H $ on the right.
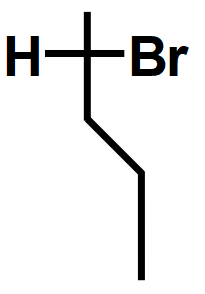
Note :
A dextrorotatory compound is prefixed with $ \left( + \right) $ or d. whereas the levorotatory compound is prefixed with the $ \left( - \right) $ or l. These lowercase are considered to be different from those of the D and L prefixes which are used in differentiating chiral configurations with the organic compounds. The carbohydrate is a biomolecule which comprises the carbon, hydrogen and the oxygen atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




