
Convert ethane into butane.
Answer
573.3k+ views
Hint: To increase the carbon chain we use a coupling reaction which can increase many numbers of carbon in a single step. In this reaction we will follow a coupling reaction involving sodium metal which will increase the carbon chain.
Complete step by step answer:
Wurtz reaction is the coupling reaction which increases the carbon chain by the number we used in reactants.
The mechanism uses two atoms of sodium in presence of dry ether.
The mechanism has two alkyl halides as their reactants.
It is a free radical mechanism.
These two alkyl halides couple with two atoms of sodium to give the desired alkane. First, we have to convert our ethane to alkyl halide or more specifically to ethyl chloride. So halogenation of alkane Is also a free radical mechanism. It is a successive replacement of hydrogen of alkane in presence of sunlight.
Halogenation of ethane
Initiation:
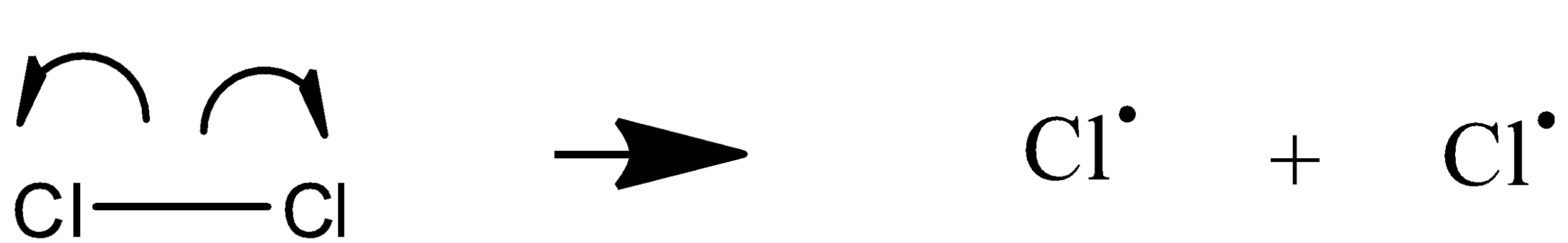
Propagation:

Termination:
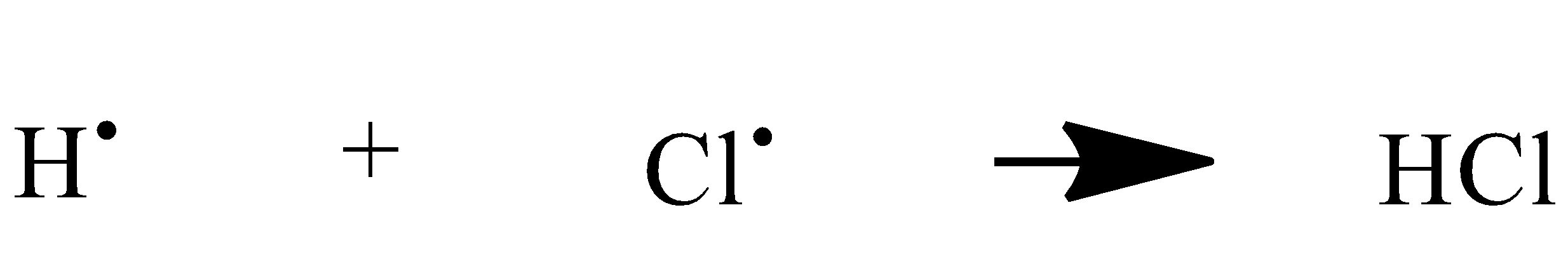
When we get our wurtz reaction ethyl chloride we will react it with sodium in presence of dry ether.
Wurtz reaction
 Ethyl chloride sodium Ethyl chloride Butane sodium chloride
Ethyl chloride sodium Ethyl chloride Butane sodium chloride
So, this is how we make butane from ethane.
Note: We use dry ether in wurtz reaction which is important as we cannot use moist ether. Moist ether contains water molecules and sodium is destructive in water as it vigorously reacts with water. So, it is mandatory to use dry ether.
Halogenation of alkyl is done by free radical mechanism which is a chain reaction. In chain reaction it is a possibility of replacing all the hydrogen with chlorine. So, Chlorine should be given in limited amounts so that only mono halide is formed.
Complete step by step answer:
Wurtz reaction is the coupling reaction which increases the carbon chain by the number we used in reactants.
The mechanism uses two atoms of sodium in presence of dry ether.
The mechanism has two alkyl halides as their reactants.
It is a free radical mechanism.
These two alkyl halides couple with two atoms of sodium to give the desired alkane. First, we have to convert our ethane to alkyl halide or more specifically to ethyl chloride. So halogenation of alkane Is also a free radical mechanism. It is a successive replacement of hydrogen of alkane in presence of sunlight.
Halogenation of ethane
Initiation:

Propagation:

Termination:
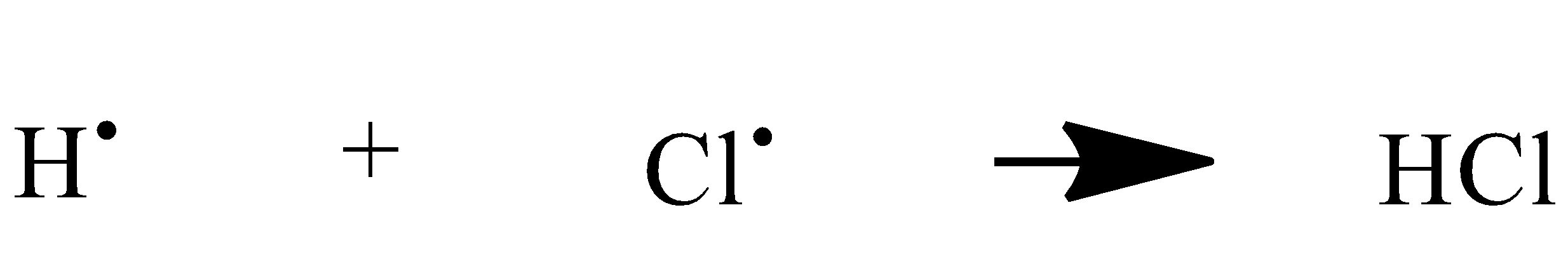
When we get our wurtz reaction ethyl chloride we will react it with sodium in presence of dry ether.
Wurtz reaction

So, this is how we make butane from ethane.
Note: We use dry ether in wurtz reaction which is important as we cannot use moist ether. Moist ether contains water molecules and sodium is destructive in water as it vigorously reacts with water. So, it is mandatory to use dry ether.
Halogenation of alkyl is done by free radical mechanism which is a chain reaction. In chain reaction it is a possibility of replacing all the hydrogen with chlorine. So, Chlorine should be given in limited amounts so that only mono halide is formed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




