
How would you convert cyclohexanol to chlorocyclohexane?
Answer
563.1k+ views
Hint: Cyclohexanol is a molecule having a chemical formula, ${{C}_{6}}{{H}_{12}}O$ . In the molecule of cyclohexane, one hydrogen atom is replaced by one hydroxyl group, which results in the formation of cyclohexanol. It is a colorless solid, which has a camphor like odour.
Complete step-by-step answer:When cyclohexanol is reacted with thionyl chloride, it results in the formation of chlorocyclohexane. The byproducts formed during this reaction are sulphur dioxide and hydrogen chloride. They are present in gaseous form and can be removed easily during the reaction. This is why this method is a preferred method when an alcohol is converted into alkyl chloride.
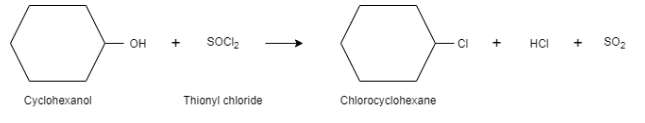
In this reaction, the hydroxyl group acts as a leaving group and chloride ion is the nucleophile. In this reaction, a bond is broken between carbon atom and oxygen atom and the new bond is formed between carbon atom and chlorine atom. It is a substitution reaction that follows the $S{{N}^{2}}$ mechanism. In the first step, the oxygen atom of cyclohexanol attacks on the sulphur of thionyl chloride, which results in the displacement of chloride ions. Here, the alcohol acts as a good leaving group.
In the second step, the chloride ion attacks the carbon, which results in the breakage of the carbon – oxygen bond. This shows $S{{N}^{2}}$ mechanism.
Note:The above reaction is a substitution reaction in which one functional group of a compound is replaced by another functional group.
The $S{{N}^{2}}$ reaction is the elimination of the leaving group and a nucleophile is added simultaneously in the reaction. $S{{N}^{2}}$ reaction only takes place when a nucleophile has the excess to the central carbon atom.
Complete step-by-step answer:When cyclohexanol is reacted with thionyl chloride, it results in the formation of chlorocyclohexane. The byproducts formed during this reaction are sulphur dioxide and hydrogen chloride. They are present in gaseous form and can be removed easily during the reaction. This is why this method is a preferred method when an alcohol is converted into alkyl chloride.

In this reaction, the hydroxyl group acts as a leaving group and chloride ion is the nucleophile. In this reaction, a bond is broken between carbon atom and oxygen atom and the new bond is formed between carbon atom and chlorine atom. It is a substitution reaction that follows the $S{{N}^{2}}$ mechanism. In the first step, the oxygen atom of cyclohexanol attacks on the sulphur of thionyl chloride, which results in the displacement of chloride ions. Here, the alcohol acts as a good leaving group.
In the second step, the chloride ion attacks the carbon, which results in the breakage of the carbon – oxygen bond. This shows $S{{N}^{2}}$ mechanism.
Note:The above reaction is a substitution reaction in which one functional group of a compound is replaced by another functional group.
The $S{{N}^{2}}$ reaction is the elimination of the leaving group and a nucleophile is added simultaneously in the reaction. $S{{N}^{2}}$ reaction only takes place when a nucleophile has the excess to the central carbon atom.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




