
How will you convert Benzene to Benzoic acid ?
Answer
609k+ views
Hint: Benzoic acid means COOH group presence. Alkylation followed by further oxidation in the presence of oxidizing agent makes it easier for the conversion of benzene into Benzoic Acid.
Complete step by step answer:
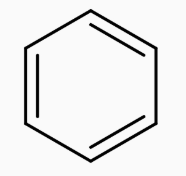
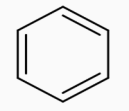
The figure represents the structure of benzene.Our First step is to convert benzene to Toluene. This can be achieved by Alkylation of benzene with $\text{CHC}{{\text{l}}_{3}}.$Along with anhyd $\text{AlC}{{\text{l}}_{\text{3}}}\text{,}$the whole process is called the Friedel Craft Alkylation Process.
$\underset{\text{anhyd}\text{.AlC}{{\text{l}}_{3}}}{\overset{\text{CHC}{{\text{l}}_{3}}}{\mathop{\to }}}\,$
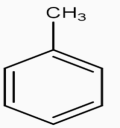
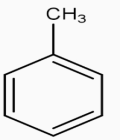
2) Next step is to convert This methyl group into COOH group by the help of strong oxidation agent $\text{KMn}{{\text{O}}_{4}},$the reaction takes place through oxidation.
 $\underset{[\text{oxidation }\!\!]\!\!\text{ }}{\overset{\text{KMn}{{\text{O}}_{4}}}{\mathop{\to }}}\,$
$\underset{[\text{oxidation }\!\!]\!\!\text{ }}{\overset{\text{KMn}{{\text{O}}_{4}}}{\mathop{\to }}}\,$
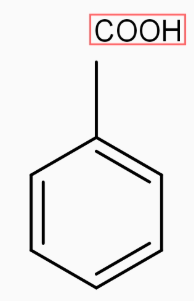 .
.
So, we finally get our final product as Benzoic Acid.
Additional Information: Benzene was first discovered by the English scientist Michael Faraday in 1825 in illuminating gas. In 1834 German Chemist Eilhardt Mitscherlich heated benzoic acid and produced benzene. Benzene is the natural constituent of crude oil and is also one of the most important elements of petrochemical. Due to its cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon.
> Chemical Properties of benzene and benzoic acid:
1) Benzene is immiscible in water but soluble in organic solvents.
2) It is colourless liquid and has an aromatic odour.
3) Benzene shows resonance.
4) It has a density of 0.87g$\text{c}{{\text{m}}^{-3}}$ .
5) Benzoic acid is an aromatic carboxylic acid.
6) Benzoic Acid has a colorless appearance in its solid state, which is of crystalline nature.
Note: We can also convert benzene into benzoic acid by the following steps also:
first convert benzene into benzaldehyde by treating benzene with CO and HCl in the presence of $\text{AlC}{{\text{l}}_{3}}$,And formylation takes place to give benzaldehyde.
Second Step: We can now convert benzaldehyde into benzoic acid in the presence of oxidizing agents. So don't confuse yourself which process should be followed, Both the processes are fine.
Complete step by step answer:

The figure represents the structure of benzene.Our First step is to convert benzene to Toluene. This can be achieved by Alkylation of benzene with $\text{CHC}{{\text{l}}_{3}}.$Along with anhyd $\text{AlC}{{\text{l}}_{\text{3}}}\text{,}$the whole process is called the Friedel Craft Alkylation Process.

$\underset{\text{anhyd}\text{.AlC}{{\text{l}}_{3}}}{\overset{\text{CHC}{{\text{l}}_{3}}}{\mathop{\to }}}\,$

2) Next step is to convert This methyl group into COOH group by the help of strong oxidation agent $\text{KMn}{{\text{O}}_{4}},$the reaction takes place through oxidation.


So, we finally get our final product as Benzoic Acid.
Additional Information: Benzene was first discovered by the English scientist Michael Faraday in 1825 in illuminating gas. In 1834 German Chemist Eilhardt Mitscherlich heated benzoic acid and produced benzene. Benzene is the natural constituent of crude oil and is also one of the most important elements of petrochemical. Due to its cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon.
> Chemical Properties of benzene and benzoic acid:
1) Benzene is immiscible in water but soluble in organic solvents.
2) It is colourless liquid and has an aromatic odour.
3) Benzene shows resonance.
4) It has a density of 0.87g$\text{c}{{\text{m}}^{-3}}$ .
5) Benzoic acid is an aromatic carboxylic acid.
6) Benzoic Acid has a colorless appearance in its solid state, which is of crystalline nature.
Note: We can also convert benzene into benzoic acid by the following steps also:
first convert benzene into benzaldehyde by treating benzene with CO and HCl in the presence of $\text{AlC}{{\text{l}}_{3}}$,And formylation takes place to give benzaldehyde.
Second Step: We can now convert benzaldehyde into benzoic acid in the presence of oxidizing agents. So don't confuse yourself which process should be followed, Both the processes are fine.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




