
How to convert aniline to fluorobenzene?
Answer
507k+ views
Hint: We have to know that, the Aniline can be changed over into fluoro benzene by first changing aniline over to benzene diazonium chloride and afterward the benzene diazonium chloride to fluorobenzene. By and large the response will be substitution of amine bunch in benzene ring by a fluorine molecule.
Complete answer:
We have to know that aniline is an aromatic compound with an $ - N{H_2}$ bunch connected to a benzene ring. In fluoro benzene, rather than this $ - N{H_2}$ bunch, a fluorine molecule is available. The transformation of aniline to fluorobenzene included two stages. Allow us to examine each progression exhaustively.
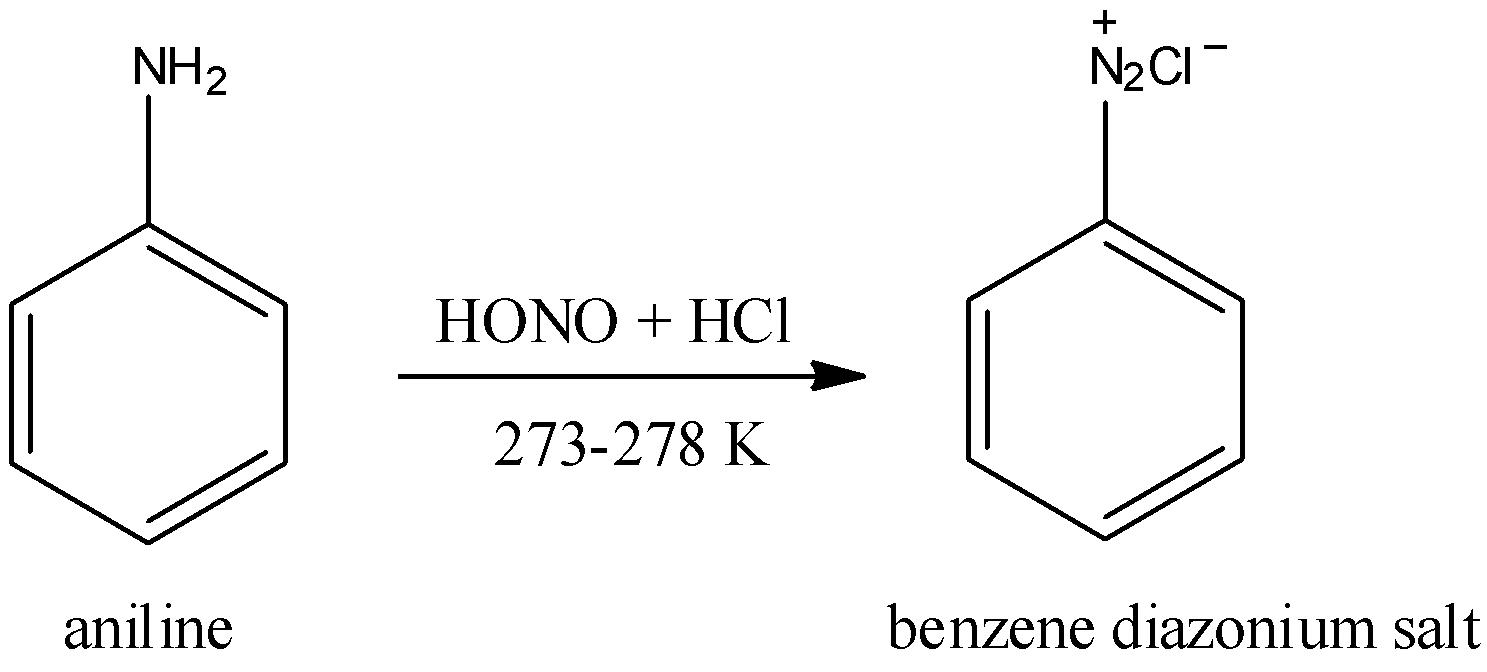
The initial step is the change of aniline to benzene diazonium chloride. At the point when aniline is blended in with weakened hydrochloric corrosive and a watery arrangement of sodium nitrate at the temperature $273 - 278K$ , benzene diazonium chloride is shaped. This response is called diazotization response. Diazotization responses are generally alluded to as the change of essential sweet-smelling amine to comparing diazonium salt. First sodium nitrate responds with $HCl$ to shape $HN{O_2}$ . It then, at that point creates an electrophile and assaults the aniline. This response is given underneath.
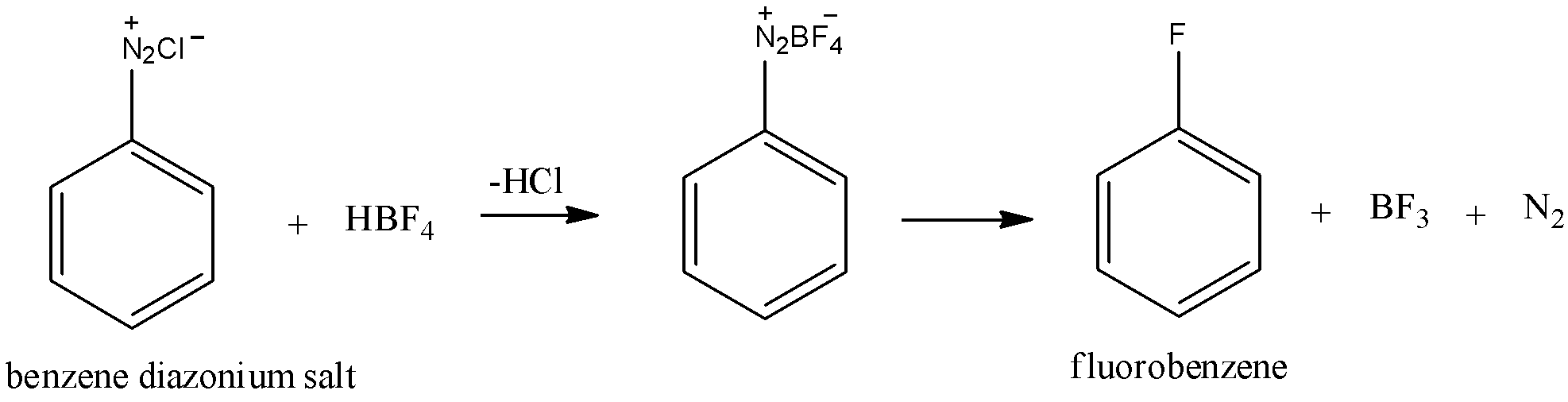
The subsequent advance is the transformation of benzene diazonium chloride to fluorobenzene. The benzene diazonium chloride frame is then treated with fluoro boric corrosive $\left( {HB{F_4}} \right)$ to yield fluoro benzene. This response additionally requires heat.
First the chloride particle in benzene diazonium chloride is supplanted with $BF_4^ - $ . The subsequent compound wipes out nitrogen gas and boron trifluoride to give fluoro benzene. This reaction must have appeared beneath.
Note:
This is a typical technique for readiness of fluoro benzene. Fluoro benzene is a dreary fluid and its solvency in water is low. Fluoro benzene acts diversely contrasted with other halobenzenes due to pi-giver properties of fluorine.
Complete answer:
We have to know that aniline is an aromatic compound with an $ - N{H_2}$ bunch connected to a benzene ring. In fluoro benzene, rather than this $ - N{H_2}$ bunch, a fluorine molecule is available. The transformation of aniline to fluorobenzene included two stages. Allow us to examine each progression exhaustively.
The initial step is the change of aniline to benzene diazonium chloride. At the point when aniline is blended in with weakened hydrochloric corrosive and a watery arrangement of sodium nitrate at the temperature $273 - 278K$ , benzene diazonium chloride is shaped. This response is called diazotization response. Diazotization responses are generally alluded to as the change of essential sweet-smelling amine to comparing diazonium salt. First sodium nitrate responds with $HCl$ to shape $HN{O_2}$ . It then, at that point creates an electrophile and assaults the aniline. This response is given underneath.

The subsequent advance is the transformation of benzene diazonium chloride to fluorobenzene. The benzene diazonium chloride frame is then treated with fluoro boric corrosive $\left( {HB{F_4}} \right)$ to yield fluoro benzene. This response additionally requires heat.
First the chloride particle in benzene diazonium chloride is supplanted with $BF_4^ - $ . The subsequent compound wipes out nitrogen gas and boron trifluoride to give fluoro benzene. This reaction must have appeared beneath.

Note:
This is a typical technique for readiness of fluoro benzene. Fluoro benzene is a dreary fluid and its solvency in water is low. Fluoro benzene acts diversely contrasted with other halobenzenes due to pi-giver properties of fluorine.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




