
Consider the following reaction,
$Phenol\xrightarrow{Zn\text{ }dust}X\xrightarrow[anhyd.AlC{{l}_{3}}]{C{{H}_{3}}Cl}Y\xrightarrow{alkaline\text{ KMn}{{\text{O}}_{4}}}Z$
The product Z is:
Answer
573k+ views
Hint: When phenol is made to react with zinc dust, it results in the elimination of the -OH group and the product X formed when treated with alkyl halide in the presence of anhydrous aluminum chloride undergoes alkylation and forms Y which when made to react with the alkaline potassium permanganate forms the product Z which is an acid. Now identify X, Y and Z.
Complete answer:
First of all, let’s discuss what is phenol. Phenols are the aromatic compounds consisting of the hydroxyl group i.e. the -OH group.
Now considering the statement;
When phenol is treated with the zinc dust it results in the formation of the benzene. The reaction occurs as;
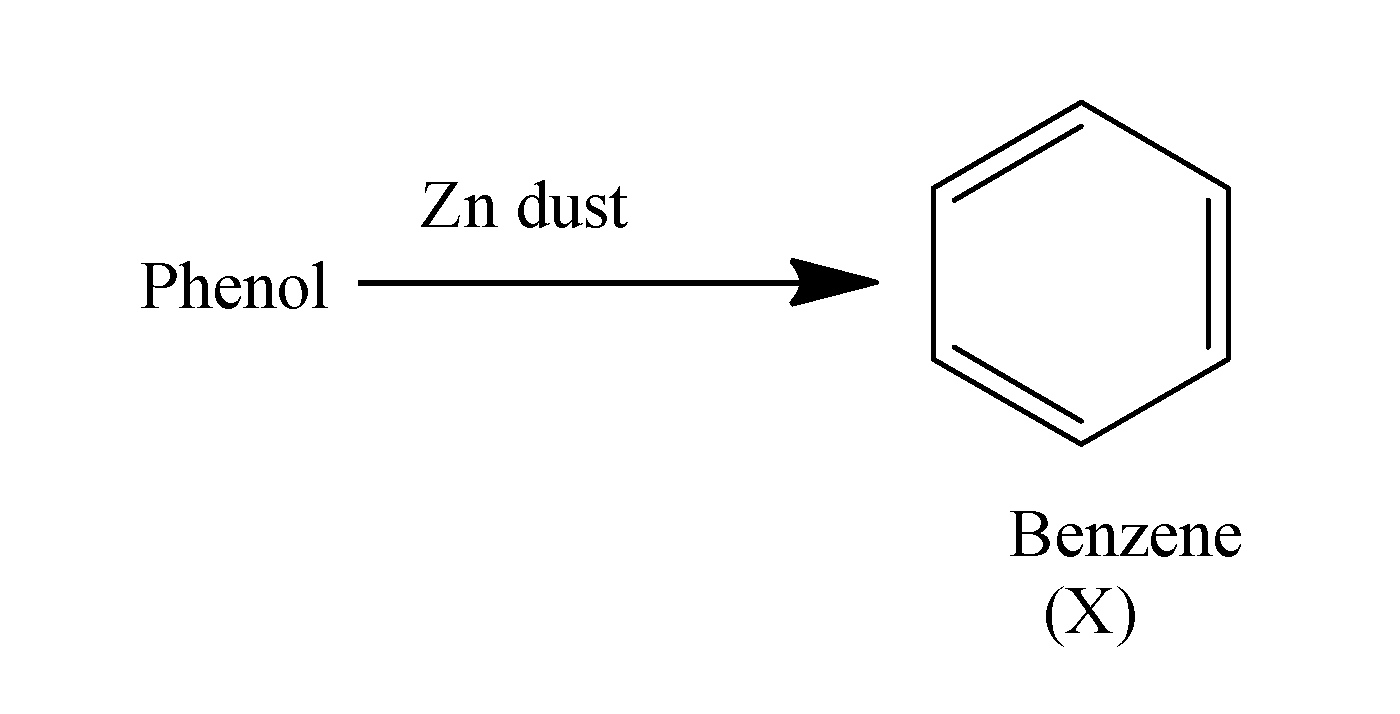
When this benzene ring is made to react with the methyl chloride in the presence of anhydrous aluminum chloride , it undergoes alkylation and results in the formation of methyl chloride i.e. toluene. The reaction occurs as;
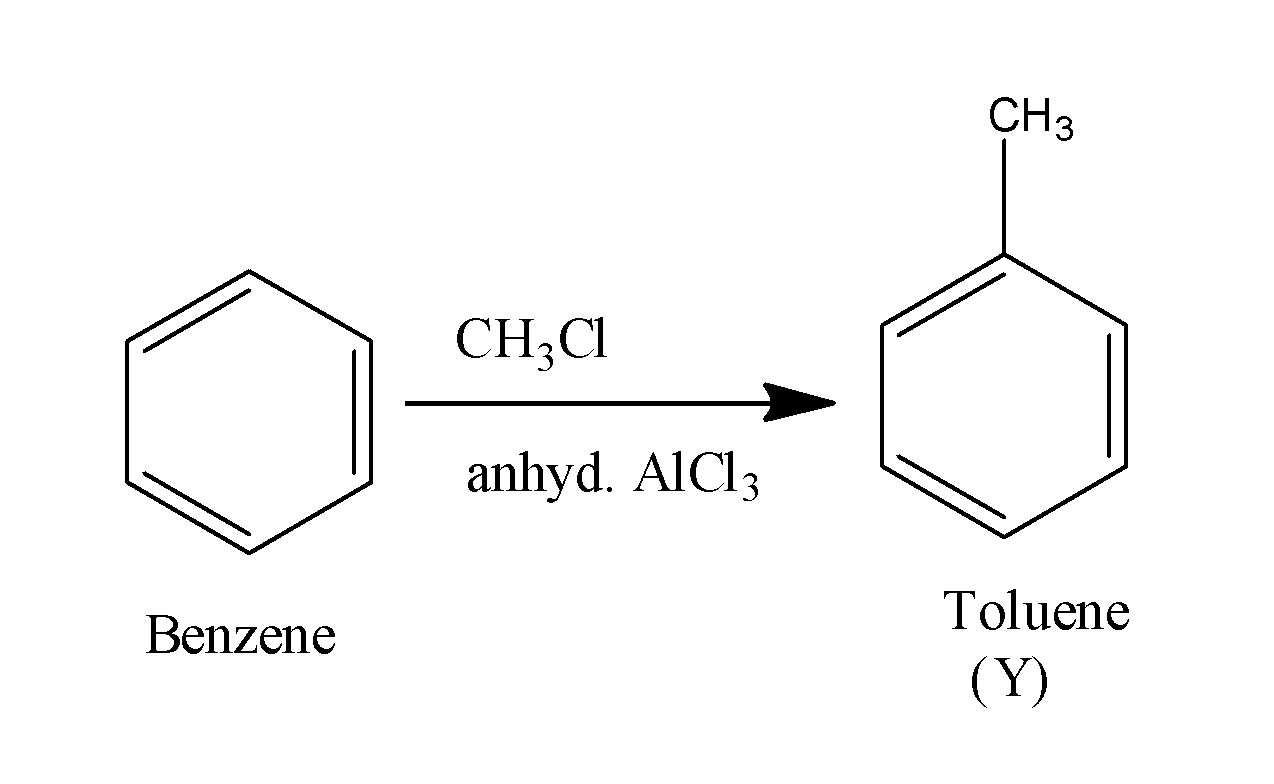
When this toluene is made to react with the alkaline potassium permanganate, it results in the formation of acid along with the removal of hydrogen gas. The reaction occurs as;
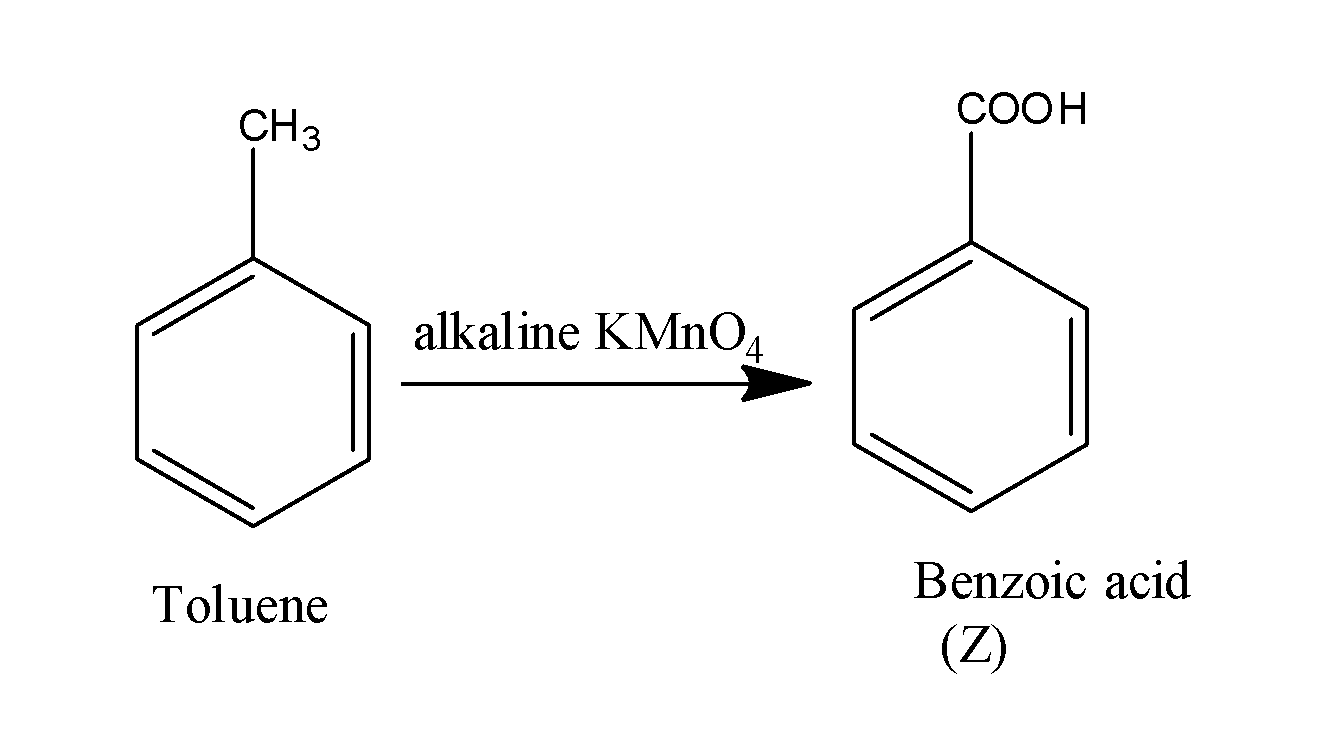
Thus, the overall reaction occurs as;
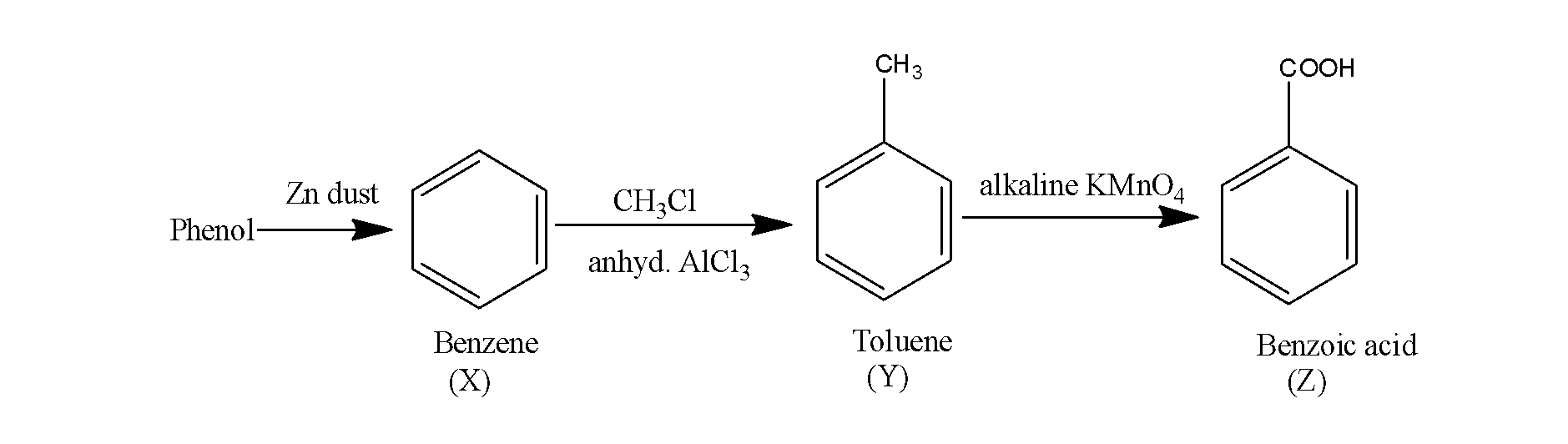
So, the product Z is benzoic acid.
Hence, option (b) is correct.
Note: The reaction of benzene with the alkyl chloride in the presence of aluminum chloride is known as the friedel craft alkylation and such reactions are known as the electrophilic substitutions reactions i.e. the hydrogen atom attached to the carbon atom of the benzene ring is replaced with the alkyl group.
Complete answer:
First of all, let’s discuss what is phenol. Phenols are the aromatic compounds consisting of the hydroxyl group i.e. the -OH group.
Now considering the statement;
When phenol is treated with the zinc dust it results in the formation of the benzene. The reaction occurs as;

When this benzene ring is made to react with the methyl chloride in the presence of anhydrous aluminum chloride , it undergoes alkylation and results in the formation of methyl chloride i.e. toluene. The reaction occurs as;

When this toluene is made to react with the alkaline potassium permanganate, it results in the formation of acid along with the removal of hydrogen gas. The reaction occurs as;

Thus, the overall reaction occurs as;

So, the product Z is benzoic acid.
Hence, option (b) is correct.
Note: The reaction of benzene with the alkyl chloride in the presence of aluminum chloride is known as the friedel craft alkylation and such reactions are known as the electrophilic substitutions reactions i.e. the hydrogen atom attached to the carbon atom of the benzene ring is replaced with the alkyl group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




