
What is the condensed structural formula for toluene with bromine?
Answer
531.6k+ views
Hint: A condensed structural formula is the representation of the structural formula but different from a structural formula. It is compact and easier to write. It consists of the general formula of any molecule, but the atoms are placed in a manner that depicts the structural position of the atoms. Toluene is a benzene ring with a methyl group at Para position.
Complete answer:
The condensed structural formulas of molecules are convenient to study and easy to write in a more compact way. The condensed structural formula for compounds are different from the chemical or molecular formula, as they consist of the atoms according to their positions as in the structural formula, while the molecular formula tells the total number of atoms present in the molecule without telling us the positions of atoms.
Toluene is a benzene ring with a $C{{H}_{3}}$ (methyl) group at Para position. We have been given a toluene with bromine atoms to detect the condensed structural formula. Now, considering that the bromine is attached at the ortho position, we will have the structure of the compound as,
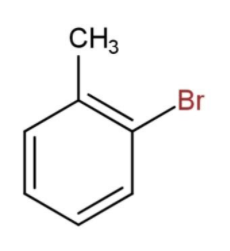
Then, the formula for toluene with bromine at ortho position will be ${{C}_{6}}{{H}_{4}}C{{H}_{3}}Br$
Hence, the condensed structural formula for toluene with bromine is ${{C}_{6}}{{H}_{4}}C{{H}_{3}}Br$.
Note:
There may be another instance when the bromine atom is attached with the methyl group of toluene then the structure becomes,
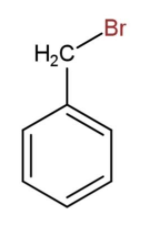
So, the formula will be ${{C}_{6}}{{H}_{5}}C{{H}_{2}}Br$. In both the cases the molecular formula will be the same as ${{C}_{7}}{{H}_{7}}Br$. In ${{C}_{6}}{{H}_{4}}C{{H}_{3}}Br$, the bromine can be attached on ortho, meta or para positions also but the condensed formula will be same.
Complete answer:
The condensed structural formulas of molecules are convenient to study and easy to write in a more compact way. The condensed structural formula for compounds are different from the chemical or molecular formula, as they consist of the atoms according to their positions as in the structural formula, while the molecular formula tells the total number of atoms present in the molecule without telling us the positions of atoms.
Toluene is a benzene ring with a $C{{H}_{3}}$ (methyl) group at Para position. We have been given a toluene with bromine atoms to detect the condensed structural formula. Now, considering that the bromine is attached at the ortho position, we will have the structure of the compound as,

Then, the formula for toluene with bromine at ortho position will be ${{C}_{6}}{{H}_{4}}C{{H}_{3}}Br$
Hence, the condensed structural formula for toluene with bromine is ${{C}_{6}}{{H}_{4}}C{{H}_{3}}Br$.
Note:
There may be another instance when the bromine atom is attached with the methyl group of toluene then the structure becomes,

So, the formula will be ${{C}_{6}}{{H}_{5}}C{{H}_{2}}Br$. In both the cases the molecular formula will be the same as ${{C}_{7}}{{H}_{7}}Br$. In ${{C}_{6}}{{H}_{4}}C{{H}_{3}}Br$, the bromine can be attached on ortho, meta or para positions also but the condensed formula will be same.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




